We just published an interview: Seren Kell on the research gaps holding back alternative proteins from mass adoption. Listen on Spotify or click through for other audio options, transcript, and related links. Below are the episode summary and some key excerpts.
Episode summary
There have been literally thousands of years of breeding and living with animals to optimise these kinds of problems. But because we’re just so early on with alternative proteins and there’s so much white space, it’s actually just really exciting to know that we can keep on innovating and being far more efficient than this existing technology — which, fundamentally, is just quite inefficient. You’re feeding animals a bunch of food to then extract a small fraction of their biomass to then eat that.
Animal agriculture takes up 83% of farmland, but produces just 18% of food calories. So the current system just is so wasteful. And the limiting factor is that you’re just growing a bunch of food to then feed a third of the world’s crops directly to animals, where the vast majority of those calories going in are lost to animals existing.
- Seren Kell
In today’s episode, host Luisa Rodriguez interviews Seren Kell — Senior Science and Technology Manager at the Good Food Institute Europe — about making alternative proteins as tasty, cheap, and convenient as traditional meat, dairy, and egg products.
They cover:
- The basic case for alternative proteins, and why they’re so hard to make
- Why fermentation is a surprisingly promising technology for creating delicious alternative proteins
- The main scientific challenges that need to be solved to make fermentation even more useful
- The progress that’s been made on the cultivated meat front, and what it will take to make cultivated meat affordable
- How GFI Europe is helping with some of these challenges
- How people can use their careers to contribute to replacing factory farming with alternative proteins
- The best part of Seren’s job
- Plenty more
Producer and editor: Keiran Harris
Audio Engineering Lead: Ben Cordell
Technical editing: Dominic Armstrong and Milo McGuire
Additional content editing: Luisa Rodriguez and Katy Moore
Transcriptions: Katy Moore
Highlights
Why alternative proteins
Seren Kell: If you look at things like animal welfare, climate change, other forms of environmental degradation, food security, and public health — things like antimicrobial resistance and zoonotic diseases or pandemics — intensive animal agriculture is one of the leading drivers, if not the leading driver, for all of these issues. And that’s not looking to change anytime soon.
Luisa Rodriguez: Right. Yeah, I am always surprised, when I learn more about the statistics, to learn that it seems like animal agriculture is just this horrible, horrible thing — that whatever problem you look at, it’s there, making things much worse, and is a pretty big share of many of these problems. Like climate change: it feels like less of a secret or unknown fact now, but I feel like a few years ago I learned about the share of carbon emissions that come from animal agriculture, and it’s just huge. It’s one of the main things — it’s not like a little side thing; it’s like a key thing — which I found kind of mind-blowing. And it seems like that’s the case for loads of issues, including animal welfare, which I care a lot about personally.
Seren Kell: Yeah. On that climate point, one study found that if we literally eliminated fossil fuels overnight, we still couldn’t meet our Paris Agreement targets unless we addressed animal agriculture globally. And this is really the untapped source of emissions — which, as you referenced, gets far less attention.
Luisa Rodriguez: I guess we agree that animal agriculture causes lots of harm, so why work on alternative proteins as opposed to other interventions?
Seren Kell: Our thinking behind this essentially is that we haven’t been very successful historically in addressing this problem through basically telling people what to eat or asking people to reduce their meat consumption. And if we look at what actually drives consumer decision making around what they choose to buy and eat, it is, for the vast majority of people: taste, price, and accessibility. It just isn’t concerns around health or climate change. Those things are accessory factors, which might nudge people if taste, price, and convenience are already being met, but most people are not compromising on those first three. And unfortunately, it just is the case that alternatives to meat right now, for the vast majority of people, are not quite there yet on those three metrics.
So I think with that being true, we need to provide something which people actually want to eat, but is far more sustainable and far more humane. And that really is the driving theory behind alternative proteins. I think alternative proteins as an intervention into reducing the harms associated with animal agriculture, it’s not a silver bullet. I think there are complementary approaches; I won’t speak to various interventions specifically targeting things like animal welfare or specifically targeting the usage of antibiotics. I think alternative proteins has an advantage, where you’re just essentially trying to displace the problem that is the cause of all of these issues, and therefore it seems a lot more scalable than other interventions. But that’s not to say that I think it’s one or the other.
How fermentation is used in alternative proteins
Seren Kell: So fermentation has been an incredibly useful method of processing and preserving food for literally thousands of years. So beer, bread, tempeh, sauerkraut, kimchi, et cetera: those all involve traditional fermentation. And now we are seeing scientists and companies applying both traditional fermentation to alternative proteins specifically, but also other ways of using microorganisms which fall under this bucket of fermentation: biomass and precision fermentation. That can be to produce very specific functional ingredients — things like fats or flavour molecules or vitamins — but also to produce actual meaty protein itself that has the same taste and texture of animal products.
One way in which it’s helpful is that it can essentially improve the taste of plant-based ingredients. To give a specific example of this: if you’re using legumes — for example, peas — as your plant-based ingredient, it can often have this kind of beany taste, this kind of strange, odd taste associated with it. That’s one way in which fermentation can come in handy. So if you ferment, let’s say, fava beans, that can reduce that beany taste. And that’s actually what one food manufacturer, Foodiq, have done. They’ve launched this fermented fava bean ingredient where they’ve used fermentation.
Here’s another example: MycoTechnology is a company that is performing traditional fermentation of things like pea or rice or chickpea. And again, because they fermented it with a particular fungus, that’s now really improved the taste profile and added tastes like meatiness and savoriness and certain umami flavours to the product.
Biomass fermentation is a similar concept: you’re feeding microorganisms whatever they need to grow. But rather than the main biomass being the plant ingredient, it’s the actual microorganism. So you’re essentially just leveraging the fact that they can grow incredibly quickly and produce a lot of high-quality protein very quickly, and actually consuming them directly.
There’s some pretty impressive examples of this. There’s another biomass fermentation company called ENOUGH Food, and with a factory that they’re currently building in the Netherlands, they’re aiming to deliver 50,000 tonnes per year — which is the equivalent of about a cow’s worth of protein every two minutes, which is very cool.
Another one that jumps to mind is a company in Finland called Solar Foods. They’re essentially using electricity, air, and water as the inputs to then produce a load of high-quality protein.
Precision fermentation is really interesting. Again, you’re using microorganisms, but you’re using them essentially as miniature cell factories to produce very specific ingredients. And this is not at all unique to alternative proteins. Just to give a couple of examples of this, a while ago, most people got their insulin from the pig industry; it was extracted from pigs. That is no longer the case. The vast majority of insulin that diabetics use is produced via essentially this process. So they’ve taken a yeast, given it the instructions it needs to produce human insulin, and then they are growing in a tank, producing lots of the insulin, and then the insulin is purified out. It is clean, it does not have to come from a pig slaughterhouse, and it’s a much more reliable process.
There have been a few really exciting examples of this. First of all, Impossible Foods essentially pioneered precision fermentation and produced heme. Heme is an ingredient which is naturally present in animal tissue. It’s in your blood and my blood. And they’ve essentially taken the instructions for producing heme and are expressing it through precision fermentation, and then using that as an ingredient in a plant-based burger. So it’s the reason that the Impossible Burger kind of bleeds and has this kind of bloody look, and it’s supposed to be responsible for that meaty taste that you have with the Impossible Burger.
Because you have total freedom to decide which proteins or which fats or which particular ingredients you want to make, you can also just make real animal proteins. To give an example of this, we’re seeing some companies taking yeast and giving them the instructions to make things like whey or casein — which are the major proteins in cow’s milk — and then using that to produce genuinely real animal-free dairy: ice cream or yoghurt or cheeses, things like that.
What makes fermentation so exciting
Seren Kell: If you just do a standard life cycle assessment of fermentation as a process, you do have massive savings in terms of land use and greenhouse gas emissions and water usage and other metrics that are important for environmental impact.
Just to give a couple of examples of this: Quorn’s fermentation-made protein has a carbon footprint 70% lower than chicken. And I should say that chickens are a hard benchmark, because chickens are incredibly efficient from an environmental perspective, compared with beef, say.
If you do a like-for-like comparison with beef, your savings are obviously going to be a lot better than compared with chicken. So if you produce whey protein via precision fermentation, that causes 97% fewer greenhouse gas emissions than if you’re taking it from a cow directly. That’s another life cycle assessment that’s been done of a particular product.
But I think it is worth talking about the fact that not only is fermentation just less resource intensive overall, but back to that point about being able to upcycle waste side streams from other industries. There is this big opportunity for broader sustainability benefits there.
So I can just give a couple examples of what that could look like in practice. For example, there’s a German fermentation startup called Mushlabs that is collaborating with a brewery to use essentially spent grain from the beer production process as the food that they then feed to their mycoprotein.
Seren Kell: Yeah, it’s really cool. We’ve actually funded a project through our Research Grant Program where researchers are taking corn husks, which again is another waste agricultural byproduct — and again using that as a potential feedstock or food for their microorganisms to consume, and just getting high-quality protein at the end of that process. And there’s a bunch more research that can be done. Oyster mushrooms, one paper suggested recently, could grow on just hydrated wood pulp, which is an incredibly abundant side stream from the paper industry. So there’s just a lot of opportunities there.
Your Quorn chicken nuggets come from a shed
Luisa Rodriguez: So another challenge is trying to figure out which microbial strains to use for fermentation, which I think basically is like, Do you use a particular kind of yeast? Do you use bacteria? Do you use some specific cell line? Can you explain what the challenge is there?
Seren Kell: Yeah, so it is exactly as you said: it’s which organism are you using as your production host in your process? One way of looking at it is precision fermentation isn’t really a new technique. In other industries it might go by different names, like “recombinant protein production” or “synthetic biology,” but it’s an established technology. And there are a bunch of what are called “workhorse strains”: go-to production hosts that are used. There’s reasons why people would start to converge on using similar strains: they start to become more familiar; people start to characterise them better; there’s fewer regulatory barriers because it’s just well understood, whereas there are barriers to commercialising new host species.
But there basically is no reason why, from a scientific perspective, we should be limited to a very small number of strains which are well characterised by other industries. We’ve slightly hit diminishing returns in terms of how much better we can make those strains. And it would just make a lot more sense to go out there and explore the hundreds of thousands, if not millions, if not more, strains that we may not even have discovered, but still could go out and find out what they’re doing and just leverage whatever biodiversity they actually have. And again, now that we have these high-throughput screening and characterisation tools, it does actually really merit essentially just recanvassing all of the known microbial species to see what their suitability would look like for this application.
Luisa Rodriguez: Cool. I’m realising I kind of have a sense of what you mean by “high-throughput”: I think you mean something like you can do a thing computationally to do it really quickly and efficiently, and get loads of results about which strains or which targets seem good. Is it something like that?
Seren Kell: That’s exactly right. You’re predefining the kinds of things that you think might be useful traits about what you’re looking for. So whether that’s the actual strain, the host organism, or whether that’s the target molecule or ingredient that you want to be producing, and then you are screening all of the data we have already — and that might be things like the genomes, the metabolisms, the known signalling pathways — within what is known of these existing microbial species out there, and seeing what comes back. So, yeah, it’s basically like comparing and contrasting different traits really quickly using computational data.
Luisa Rodriguez: So it sounds like the challenge here, which I guess is also just an opportunity, is that there are probably billions of bacteria, fungi, yeast out in the world, and we can just look at all of them and be like, “Are any of these creating the kinds of things we need to make alternative proteins taste better or make an ingredient more cheaply?” So let’s just explore them all and find the best ones. Which is just pretty amazing.
Seren Kell: There’s a couple of nice examples here actually. One kind of fun example of this is actually the story of Quorn, the mycoprotein that is used in the Quorn company’s products. Essentially in, I believe it was the ’70s, they were like, “Let’s go and find some fungal strain that we might be able to use as a food source.” And they went out and did one of these screening approaches to go and test in different environments: Can we find a cool fungus that might be able to grow, and might have really great nutrition, and grow relatively efficiently high-protein content, and all the rest of it? And the final strain — the fusarium strain that was ultimately used, and then was selected and improved upon — I think it was found at the bottom of the garden in a shed of one of the researchers who went out and did this. And that was almost half a century ago, if I’m getting the dates right. And that is the strain that Quorn uses.
Who’s to say there aren’t many others, and many better ones out there that we just haven’t discovered yet in biology? And fungi especially are just amazing and can be very well adapted to quite extreme environments. There are certain microorganisms that live at the bottom of the sea and so can deal with really high temperatures, or can consume as their food quite strange sources. They can be very flexible, which means we could feed this thing methanol or hydrogen or carbon dioxide, and it will just turn that into protein because — through totally independent evolution elsewhere — it’s evolved to just do some really cool chemistry.
The path to affordable cultivated meat
Seren Kell: The techno-economic assessment that I mentioned from CE Delft did identify that if there were the right public investment going into the sector and solving some of those technical challenges around reducing media costs and things like that, it showed that it could be possible to bring cultivated meat production costs down to about €4.68 per kilo by the year 2030.
It shows that there is a pathway to get there, but it is contingent on this big “if” of public investment. If you look at where solar panels were in the 1990s, it exists, and for the sector more generally, they were available for very eco-conscious customers who are willing to pay a premium. But until there is significant public investment going into scaling up and solving some of those technical challenges, you won’t be able to have something which is genuinely accessible to most consumers and therefore making it a real displacement of the actual market.
Right now we have a roadmap for what making progress would look like, and what addressing the current uncertainty would look like. So we know enough to know that it is absolutely worth pursuing, and to know the scale of investment that needs to go in to pursue it. And we know enough to know that it’s not a writeoff entirely as a technology. But yeah, there is just a lot of uncertainty as to how to get there, and how to get that support from governments to get the sector there.
Luisa Rodriguez: How much funding do governments need to commit in order to get that research done?
Seren Kell: One report from the Rockefeller Foundation and BCG estimated that every single year, alternative proteins have an unmet funding need of around $40 billion. I think if you look at R&D budgets for companies, $40 billion at a global scale is actually a drop in the water compared to what is spent on other technologies.
Luisa Rodriguez: Got it. So we need people on the policy side convincing governments that that is a commitment they should be making.
Seren Kell: Yes. And I should say, crucially, there needs to be much more research funding going into the space, but there is also a very important role of scientists in continuing to flesh out and detail that roadmap for what the highest priority research areas and most promising avenues are to be going down. So we certainly need far more scientists moving into that space as well, but that is a function of research funding, and scientists don’t do research if there isn’t funding to pay for it.

I am extremely pro alternative proteins (see e.g. here) but I think we still need to be more honest about the climate impacts of agriculture, both in terms of epistemic hygiene but also in terms of argumentative strategy (I don’t think we need to exaggerate the case for APs – the case is good already! – and by exaggerating some claims we are making the whole thing less believable).
In the beginning of the interview it is discussed as a huge, huge contributor to climate change, a major driver, without presenting any numbers.
The exact numbers would depend on the choice of global warming potential (essentially: what timeframe of warming impact to care about?), I discuss this in a bit more detail here, but on typical metrics the impact of animal agriculture would be something like ~15%, see e.g. here from OWID; there’s arguments for both more optimistic and more pessimistic (caring less about short-term warming than the underlying OWID data) numbers, but I think 15% seems pretty okay as a prior before weighing them all in detail:
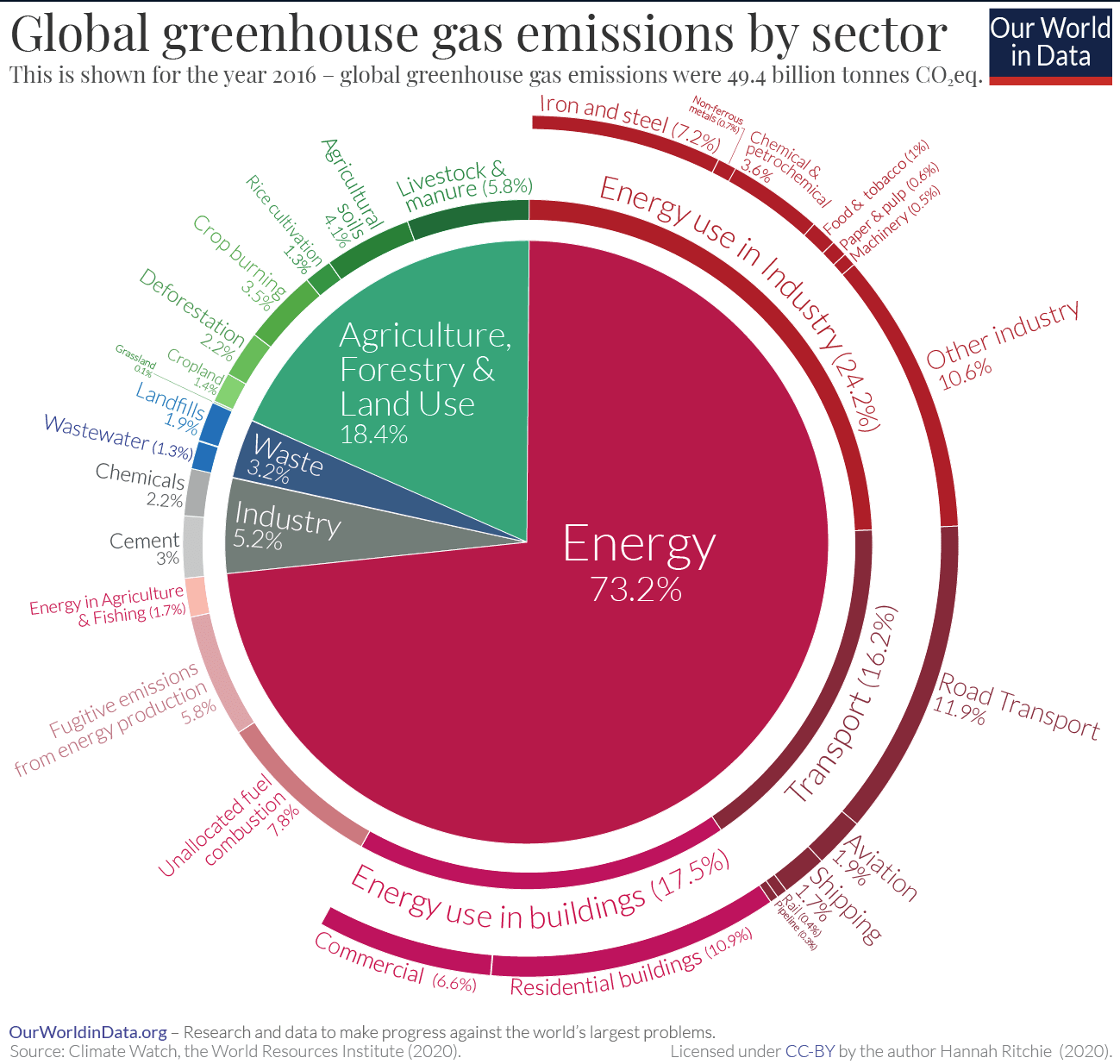
This is quite significant but if I listen to the interview and to similar messaging I would be surprised it is “only” 15%. I think it would be more honest and more robust if we said something like “alternative proteins are a promising strategy for an otherwise hard-to-decarbonize sector” (which is very exciting, few hard-to-decarbonize sectors have such promising technological solutions!) but not to suggest that it is anywhere close to the importance of transforming our energy system (~75/15 - a 5x difference).