Summary
- Hepatitis B is a highly prevalent and consequential viral infection. Hepatitis B was responsible for approximately 18.2 million DALYs in 2019. It has a global prevalence of ~4%.
- Globally, rates are improving. The African region has an under 5 prevalence rate of 2.7% and is a potential geographical focus area to scale up preventative efforts.
- Approximately 35-50% of cases of chronic Hep B are acquired as a result of mother to child transmission during delivery.
- A rapid diagnostic test is available for HBV. A cheap and effective vaccination is available to prevent mother-to-child transmission. Anti-viral medication are effective in avoiding complications of chronic infection and preventing mother-to-child transmission.
- Vaccination, especially birth dose vaccination, is under utilised, especially in the African region.
- Scaling up interventions to prevent mother-to-child transmission, such as birth dose vaccination or peripartium antiviral prophylaxis, in high prevalence regions could be particularly cost-effective, especially if layered on or implemented alongside other maternal health interventions.
Key Uncertainties
- Studies of global prevalence, complications and DALYs associated with hepatitis B are based off modelling, and there may be greater uncertainty around figures in areas where healthcare data is lower quality.
- The Cochrane review on interventions to prevent mother-to-child transmission may have overrepresented infants born to HBeAg positive mothers (as e antigen status was not reported in a number of the included studies), these results need to be interpreted with caution.
- There is little data available on the impact of HBV birth dose vaccine alone, independent of the 3 dose vaccine schedule recommended by WHO.
A note on terminology
Hepatitis B is associated with confusing terminology.
- HBsAg refers to hepatitis B surface antigen, refers to current hepatitis B infection.
- HBeAg refers to hepatitis B e antigen, refers to current infection, active viral replication and a high infectivity state.
- HBV DNA is a quantitative measure of virus in the blood.
What is Hepatitis B?
Hepatitis B is a disease caused by the Hepatitis B Virus (HBV). It can cause both an acute, and chronic hepatitis (liver infection). It is transmitted via body fluids (blood, saliva, semen or vaginal fluid) from an individual with an active viraemia (HBV circulating in the blood). Transmission can occur horizontally (from person to person) or vertically (from mother to child). Neonates and infants are most susceptible to progress to chronic infection when exposed to HBV, this most commonly occurs during delivery to a mother with active hepatitis B. Acute hepatitis B rarely requires specific treatment and is cleared in that majority of immunocompetent adults. Chronic hepatitis B (CHB) risks progressing to cirrhosis (liver scarring and failure), and CHB related liver cancers, which account for the majority of the burden of disease associated with hepatitis B. Hepatitis B is a vaccine preventable illness, delivered as a 3-4 dose regimen with a ‘birth-dose’ given to a neonate soon after delivery. Treatment in the form of anti-viral medications are available to suppress viral replication in those with established CHB, this prevents complications but rarely cures the disease.
Approximately 316 million people were infected chronically with HBV in 2019. There were approximately 331 000 deaths from HBV related cirrhosis, and 192 000 deaths from HBV related liver cancer.1 Hepatitis B resulted in approximately 18.2 million DALYs in 2019.2 The World Health Organisation in 2022 set a goal of elimination of AIDS, viral hepatitis and sexually transmitted infections by 2030. WHO has additionally focussed on a ‘triple-elimination’ initiative, focussing on the elimination of mother-to-child transmission (MTCT) of syphilis, HIV and HBV, given their shared modes of transmission and epidemiological risk factors.
Importance
What is the natural history of Hepatitis B?
Infection with HBV is virologically and immunologically complex. Infection can be conceptually delineated by a range of serological, virological and biochemical factors into phases of illness. Individuals exposed to HBV may develop an acute phase of the illness, related to viral replication in the host’s hepatocytes (liver cells). Clinically this may manifest as an acute hepatitis (presenting with liver pain, fever and jaundice), or may cause a mild non-specific illness. Acute illness is typically self-limiting with only supportive treatment required. There is a ~1% risk of acute illness progressing to acute liver failure.3 Acute liver failure has a mortality of 65-85%.4
CHB is defined as serological positivity >6 months after disease onset. Chronic infection causes morbidity and mortality by two main mechanisms, increasing the risk of cirrhosis, and HBV related liver cancers. Approximately 15-40% of those with untreated CHB will progress to cirrhosis or liver cancer.5 Development of cirrhosis and liver cancer is a result of prolonged viral replication and an associated inflammatory response. Cirrhosis and liver cancer take approximately 10-20 years to develop.4 Treatment with anti-viral medication and viral suppression reduces the risk of complication from chronic infection significantly.
Not all individuals exposed to HBV will go on to develop CHB. The risk of progressing to CHB after exposure is inversely related to age at time of first exposure.6 Neonates have an 40-90% risk of progression if born to a Hep B positive mother (can be >95% if mother is HBeAg positive), children <6 have an approximate 30% risk, and healthy adults have a risk of approximately <5%.7 It is postulated that approximately 35-50% of all CHB cases worldwide are due to mother to child transmission.8
The exact mechanism of vertical transmission is not entirely clear. Intrapartum or peripartum (during or around the time of delivery) is thought to be the predominant mechanism by which HBV is transmitted from mother to child. There may be a smaller role on intrauterine transmission (during pregnancy, prior to delivery). There is insignificant evidence to suggest transmission via breast milk.4
Hepatitis B requires serology for diagnosis. There are a range of serological markers tested for which can help clinicians differentiate current infection, past or cleared infection, vaccination, or susceptibility if exposed. If chronic infection is suspected further investigation including viral load and genotyping can be performed, as well as investigation for complication of disease. Rapid diagnostic testing (RDT) is emerging as an easy to interpret, cheap and quick alternative to serological testing in resource constrained settings. Specificity for RDTs is 98.3 to 99.3%, and sensitivity is >99% for HBsAg.9 Whilst only providing information on HBsAg status (presence or absence of HBV in the blood), they offer an accessible alternative to formal serology in resource constrained settings.
What is the burden of Hepatitis B worldwide?
Based on available seroprevalence data, it is estimated that 4.1% (95% uncertainty interval (UI) 3.7-4.5%) of the global population had chronic Hep B infection in 2019. The rate varied widely across regions. The WHO Western Pacific region had a prevalence of 7.1% (95% UI 6.3 to 7.9), the African region had a prevalence of 6.5% (5.8 to 7.3). The European region had a prevalence of 1.1% (95% UI 1.0 to 1.2). Seroprevalence of children under 5 globally was 1.0% (95% UI 0.8 to 1.2). All WHO regions had an under 5 seroprevalence of <1%, except the African region at 2.7% (95%UI 2.2 to 3.2).6
HBV was responsible for approximately 18.2 million DALYs in 2019.2 Of these, 70% were attributable to 10 countries (in order: China, India, Indonesia, Nigeria, Pakistan, Egypt, Thailand, South Korea, Bangladesh, Myanmar). HBV related disease (acute hepatitis, cirrhosis and liver cancer related to Hep B) was responsible for approximately 555 000 deaths (95% UI 487 000 to 630 000) in 2019. HBV related cirrhosis was responsible for 59.6% (95% UI 50.3 to 70.6) of deaths, HBV related liver cancer was responsible for 34.6% (95% UI 29.2 to 40.4) and acute Hep B was responsible for 5.9% (95% UI 4.3 to 8.1) of Hep B related deaths.6
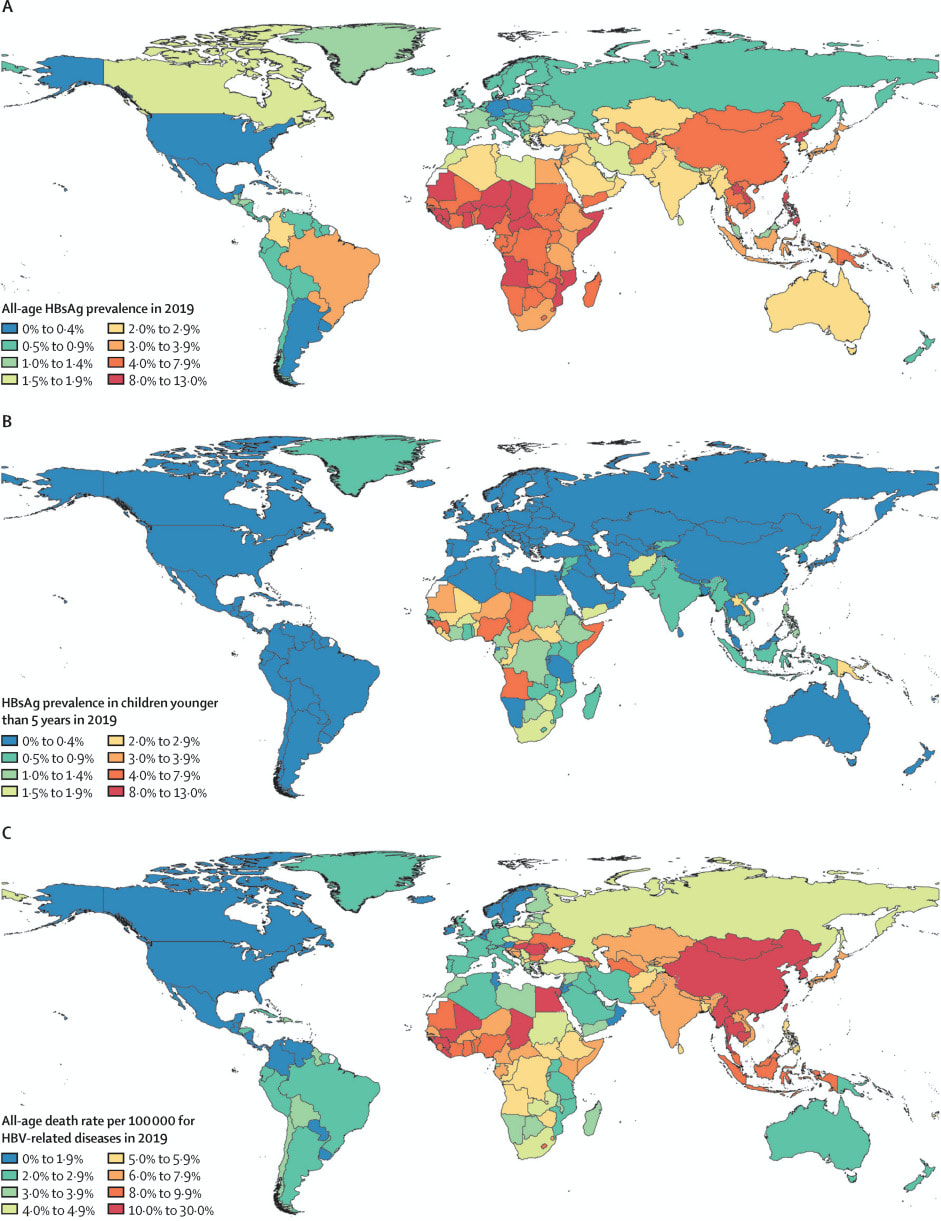
Overall rates of chronic Hep B are improving. Global, all age prevalence has decreased 31.3% (95% UI 29.0 to 33.9) since 1990. For children under 5, globally the prevalence has reduced by 76.8% (76.2 to 77.5) since 1990. The Western Pacific region had an impressive 93.6% (95% UI 93.3 to 94.0) reduction in under 5 prevalence since 1990. Since 1990, HBV related deaths increased by 5.9% (95% UI -5.6 to 19.2). Otherwise, age-specific DALY rates have decreased over time.
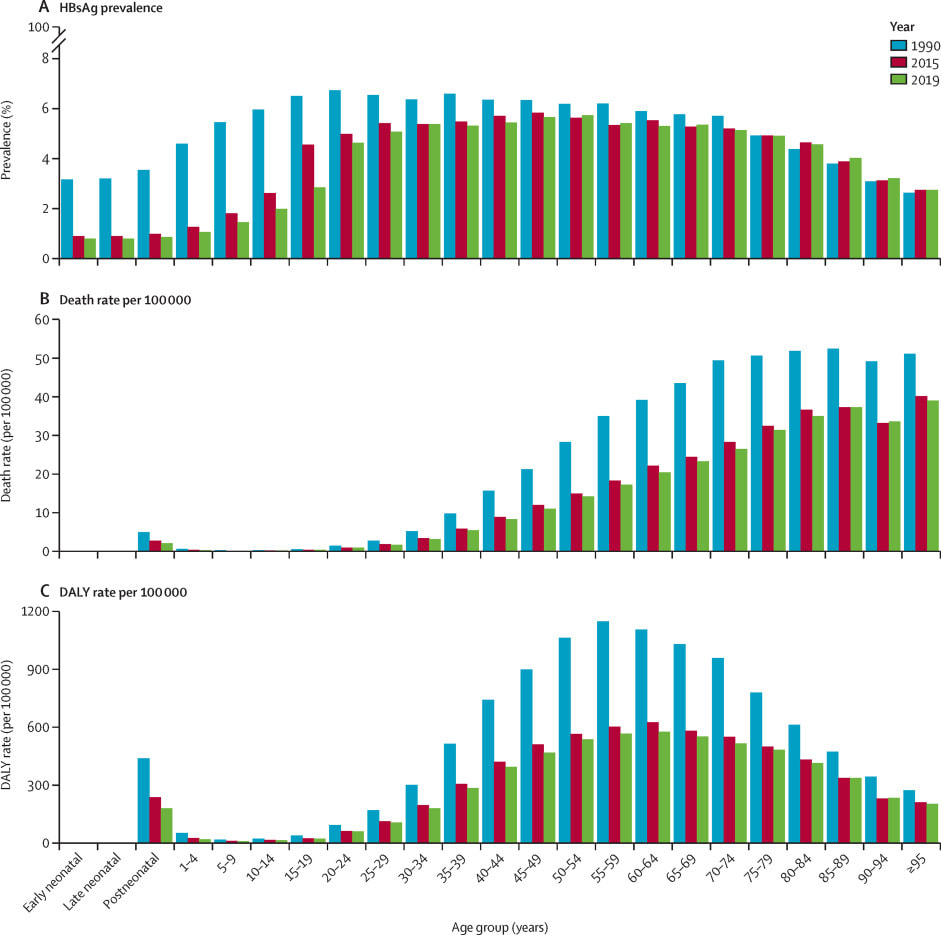
The significant declines in prevalence of hepatitis B, especially amongst children under 5, are likely linked to the scaling up of HBV vaccination in children.10 Coverage with 3-dose vaccination regimens for HBV have increased from 29% to 81% from 2000, to 2019.However there remains significant disparities globally in vaccination rates.11 The African region remains below targets with regard to vaccination rates, with less than 10% of neonates receive timely birth-dose vaccination.12
Tractability
What treatment/prevention is available for Hepatitis B?
Hepatitis B is a preventable, and treatable disease. Prevention includes vaccination, and post-exposure prophylaxis with hepatitis B immunoglobulin. Treatment of chronic infection is with anti-viral medications, which are usually continued lifelong.
Prevention
Hepatitis B vaccination and post-exposure prophylaxis with HBV immunoglobulin are both effective means of preventing CHB in infants born to HBV positive mothers. Compared to placebo, full course HBV vaccine reduced the risk of CHB in infants by approximately 72% (Risk Ratio 0.28 95% CI 0.20 to 0.40). There was no difference between 3 or 4 dose regimens. There was no difference between HBeAg positive or HBeAg negative studies included in the review. HBV immunoglobulin alone (i.e. without vaccination) reduced risk of transmission by 50% (RR 0.50 95% CI 0.41 to 0.60). Combination vaccination and HBV immunoglobulin significantly reduced the occurrence of HBV in infants by 92% (RR 0.08 95% CI 0.03 to 0.17).13
Treatment with anti-viral medication of women with HBV (peripartum antiviral prophylaxis) is strongly recommended. Anti-viral treatment reduced the risk of CHB in infants born to mothers who were HBsAg positive by 70% (RR 0.3 95% CI 0.3 to 0.4).14
Vaccination against HBV is highly cost effective. One study from The Gambia found a cost of $47 USD (2002 USD) per DALY averted.15 A modelling study which investigated the costs associated with a cold temperature chain outreach vaccine delivery model to improve acquisition of birth-dose vaccination found a cost of $0.15 to $79.72 per DALY averted from the delivery model.16 Little cost-effectiveness data is available for HBV immunoglobulin. Given it is a blood product that requires significant infrastructure and expertise at a high cost, its cost-effectiveness is unlikely to be competitive with vaccination.
A high-quality modelling study published in The Lancet Gastroenterology and Hepatology found scaling up peripartum antiviral prophylaxis to all HBsAg positive women could avert approximately 13.5 million DALYs (95% UI 12.3 million to 14.1 million) by 2100. Of these, approximately 8.3 million DALYs would averted in the African region. The cost per DALY averted ranged from $985 USD (95%UI 878 to 1128) in the African region, to $6587 USD (95% UI 5511 to 7730) in the European region.17
Treatment
Treatment of CHB is complex and guidelines vary internationally with regards to timing and choice of commencing anti-viral medication. Generally, treatment is lifelong, with <1% of individuals clearing chronic infection and being able to successfully remain off anti-viral medications.18 Treatment with commonly used anti-viral medications (e.g. entecavir and tonofovir disoproxil fumarate) can achieve viral suppression in >90% of cases.19
The cost effectiveness of anti-viral treatment varies depending on context. One cost-effectiveness modelling study found a cost of $14 590 USD per QALY gained from treatment in all HBsAg positive patients in China.20 An economic analysis from The Gambia found a cost of approximately $511 USD per QALY gained from community based screening and treatment.21
Neglectedness
Current funding
The morbidity and mortality associated with hepatitis B is largely preventable through prevention and treatment efforts. Despite improvements in rates of hepatitis B, it remains the cause of significant morbidity and mortality worldwide. Hepatitis B can be considered a neglected tropical disease insofar as it:
- Has the majority of morbidity and mortality borne by low- and middle-income countries where resources are inherently constrained and directed to competing health concerns such as HIV and malaria.
- Disproportionately impacts populations affected by poverty, causing morbidity and mortality including stigma and discrimination.
- Relatively neglected by research (i.e. resource allocation not in keeping with burden of the disease)
- Amenable to broad control, elimination or eradication by applying public health strategies.22
A review of expenditure on infectious disease research in the UK from 1997 to 2013 found 0.7% of total expenditure allocated to hepatitis B, 13.9% allocated to malaria, and 17.5% allocated to HIV. This is incommensurate with the relative burden from each of the respective diseases.23
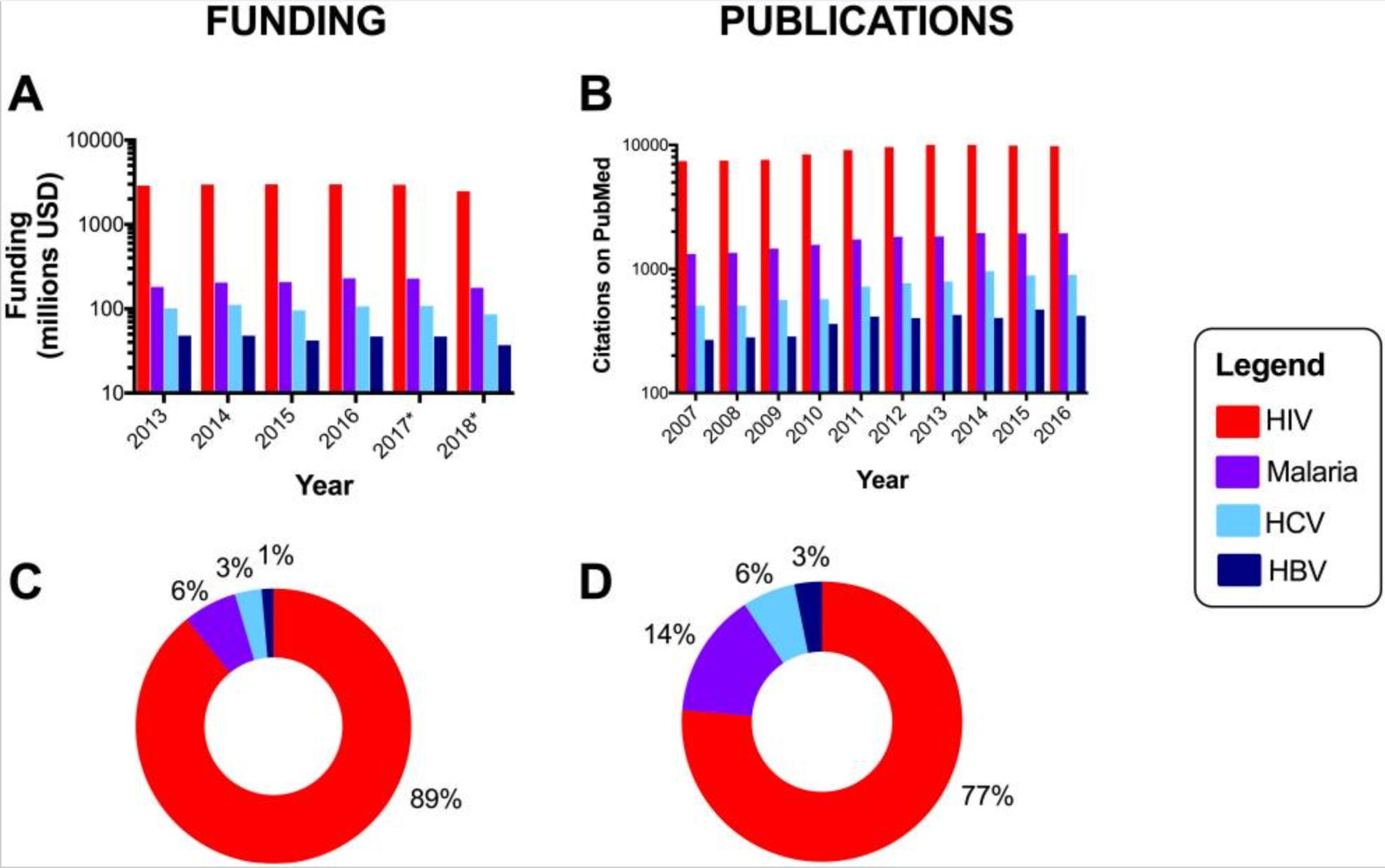
More anecdotally, when conducting an initial search on Medline for a recent systematic-review on interventions to improve screening for maternal HIV, syphilis and HBV, a sensitive search approach yielded 1 288 results for HIV, 457 results for syphilis, and only 160 results for hepatitis B. This is despite HIV (all ages) resulting in 47.6 million DALYs,24 congenital syphilis resulting in 3.6 million DALYs,25 and hepatitis B resulting in 18.2 million DALYs.6
There are definite gaps in the landscape of hepatitis B, especially for interventions with proven effectiveness. Scaling up of programs to ensure antenatal screening, birth dose hepatitis B vaccination, and potentially HBV peripartum antiviral prophylaxis offer cost-effective means of addressing mother-to-child transmission. Statistics on rates of antenatal hepatitis B screening are sparse, a review from Nigeria found a screening rate of 7.2% for hepatitis B, compared with 16.3% for syphilis and 90.3% for HIV.26 Although global coverage rates for hepatitis B vaccination is approximately 80%, only 42% receive a birth dose vaccination. In the African region, the birth dose vaccination rate is approximately 17%.27 Peripartum antiviral prophylaxis, although recommended by WHO, has an estimated uptake rate of <1% in eligible women.28
References
1. Hsu Y-C, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nature Reviews Gastroenterology & Hepatology 2023: 1-14.
2. IHME. Global Burden of Disease Study 2019 (GBD 2019). 2021. https://vizhub.healthdata.org/gbd-results.
3. Shiffman M. Management of acute hepatitis B. Clin Liver Dis 2010; 14(1).
4. Batra Y, Acharya S. Acute liver failure: prognostic markers. Indian J Gastroenterol 2003; 22 Suppl 2.
5. McMahon B. Natural history of chronic hepatitis B. Clin Liver Dis 2010; 14(3).
6. Collaborators G. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022; 7(9).
7. Akseer N, Rizvi A, Bhatti Z, et al. Association of Exposure to Civil Conflict With Maternal Resilience and Maternal and Child Health and Health System Performance in Afghanistan. JAMA Netw Open 2019; 2(11): e1914819.
8. Yao JL. Perinatal transmission of hepatitis B virus infection and vaccination in China. Gut 1996.
9. Chevaliez S, Roudot-Thoraval F, Hézode C, Pawlotsky J, Njouom R. Performance of rapid diagnostic tests for hepatitis B surface antigen detection in serum or plasma. Diagnostic microbiology and infectious disease 2021; 100(2).
10. Cui F, Shen L, Li L, et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg Infect Dis 2017; 23(5).
11. GBD Collaborators VCC. Measuring routine childhood vaccination coverage in 204 countries and territories, 1980-2019: a systematic analysis for the Global Burden of Disease Study 2020, Release 1. Lancet 2021; 398(10299).
12. Lesi O, Ward J. Paving the way towards hepatitis B virus-free generations in Africa. Lancet Glob Health 2021; 9(11).
13. Lee C, Gong Y, Brok J, Boxall E, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev 2006; (2).
14. Brown R, McMahon B, Lok A, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 2016; 63(1).
15. Kim S, Salomon J, Goldie S. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ 2007; 85(11).
16. Scott N, Palmer A, Morgan C, et al. Cost-effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: a modelling study. Lancet Glob Health 2018; 6(6): e659-e67.
17. Nayagam S, de Villiers M, Shimakawa Y, et al. Impact and cost-effectiveness of hepatitis B virus prophylaxis in pregnancy: a dynamic simulation modelling study. Lancet Gastroenterol Hepatol 2023; 8(7).
18. Wu Y, Shen C, Chen X. Antiviral treatment for chronic hepatitis B: Safety, effectiveness, and prognosis. World J Clin Cases 2019; 7(14).
19. Scaglione S, Lok A. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology 2012; 142(6).
20. Zhang S, Wang C, Liu B, et al. Cost-effectiveness of expanded antiviral treatment for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Reg Health West Pac 2023; 35.
21. Nayagam S, Conteh L, Sicuri E, et al. Cost-effectiveness of community-based screening and treatment for chronic hepatitis B in The Gambia: an economic modelling analysis. Lancet Glob Health 2016; 4(8).
22. O'Hara G, McNaughton A, Maponga T, et al. Hepatitis B virus infection as a neglected tropical disease. PLoS Negl Trop Dis 2017; 11(10).
23. Head M, Fitchett J, Nageshwaran V, Kumari N, Hayward A, Atun R. Research Investments in Global Health: A Systematic Analysis of UK Infectious Disease Research Funding and Global Health Metrics, 1997-2013. EBioMedicine 2015; 3.
24. Tian X, Chen J, Wang X, et al. Global, regional, and national HIV/AIDS disease burden levels and trends in 1990-2019: A systematic analysis for the global burden of disease 2019 study. Frontiers in public health 2023; 11.
25. Kahn J, Jiwani A, Gomez G, et al. The cost and cost-effectiveness of scaling up screening and treatment of syphilis in pregnancy: a model. PloS one 2014; 9(1).
26. Olakunde BO, Adeyinka DA, Ndukwe CD, Oladele TT, Yahaya HB, Ijaodola OA. Antenatal hepatitis B screening in Nigeria: A comparative analysis with syphilis and HIV. Int J STD AIDS 2021; 32(14): 1290-7.
27. WHO. Hepatitis B vaccination coverage. 2024. https://immunizationdata.who.int/pages/coverage/HEPB.html?CODE=Global&ANTIGEN=&YEAR= (accessed 16 Feb 2024).
28. Polaris. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3(6).
