This report was conducted within the pilot for Charity Entrepreneurship’s Research Training Program in the fall of 2023 and took around eighty hours to complete (roughly translating into two weeks of work). Please interpret the confidence of the conclusions of this report with those points in mind. Please also note that the intervention was assigned to me for this report.
For questions about the research process, please contact Leonie Falk at leonie@charityentrepreneurship.com. For questions about the content of this research, please contact me directly.
The full report can also be accessed as a PDF here.
Thanks to Leonie Falk, Morgan Fairless and Priyansha Bajoria for their review and feedback. I am also grateful to the experts who took the time to offer their thoughts on this research.
Executive summary
Rabies is an infectious disease primarily transmitted to humans through dogs. The disease is estimated to cause up to 60,000 deaths per year and is 100% fatal if untreated.
While effective rabies treatment is available in many countries, the WHO, FAO and other organizations have emphasized prevention through dog vaccination as a particularly promising way to alleviate the burden of disease from rabies. While a lot of dog vaccination campaigns rely on parenteral vaccination, oral rabies vaccination is particularly well-suited to target dogs that are difficult to approach as they are delivered as baits for dogs to eat.
The evidence base for this intervention turns out to be quite weak. While there are several studies which look at oral vaccinations campaigns’ effectiveness in terms of vaccinating a large proportion of a region’s dog population, there is no evidence on the link between rabies vaccines and reduction in human rabies cases and deaths, and only weak evidence on the reduction on dog rabies incidence and human dog-bite incidence.
Experts took a negative view of a new organization working on direct delivery since rabies control campaigns are only permanently effective as an (inter-)national effort with substantial follow-up surveillance activity.
The cost-effectiveness analysis of a hypothetical five-year intervention in India results in an estimated $54,268 per averted DALY. Even under the most optimistic assumptions, the intervention would not be cost-effective, nor have a large impact in terms of lives saved.
Overall, my view is that oral rabies vaccination for dogs is not a recommendable intervention.
1 Background
1.1 Rabies transmission and symptoms
Rabies is a viral infectious disease transmitted through direct contact (i.e., through broken skin, or eye, nose and mouth mucosa) with saliva from an infected animal. Usually, people get rabies from a bite from a rabid animal, and in rare cases from non-bite injuries such as scratches. Other kinds of contact, such as petting a rabid animal or contact with blood, urine or feces, does not lead to rabies transmission (CDC, 2019). Nowadays, around 99% of global human rabies cases are transmitted by dogs (WHO, 2023), a large proportion of these dogs being free-roaming (WOAH, 2022).
When an animal or human is bitten by a rabid animal, the infected saliva enters the wound and travels through the nerves to the spinal cord and brain. This incubation period can last between several weeks to months. During this time, the infected animal or person shows no symptoms and cannot transmit the virus to others. Once the virus reaches the brain, it multiplies and passes to the salivary glands. This is when the human or animal starts to show symptoms, which come in two forms: furious rabies (including symptoms such as hyperactivity, aggression and hallucinations) and paralytic rabies (including symptoms such as paralysis and coma) (WHO, 2023). Once symptoms appear, treatment is no longer possible, and the human or animal dies within about a week (CDC, 2017).
1.2 Global burden of disease
The exact burden of disease from rabies is unclear, since different sources provide different numbers and estimates. IHME (2019) reports 13,700 deaths (95% CI 6,020–17,900) and 782,000 (320,000–1,080,000) DALYs for the year 2019. In contrast, Hampson et al. (2015) report 59,000 deaths (95% CI 25,000–159,000) and 3,700,000 (1,600,000–10,400,000) DALYs. Some older studies also report numbers between 50,000–60,000 human rabies deaths per year (Haupt et al., 1999, Knobel et al., 2005).
According to Our World in Data (2019) (which uses the data from IHME (2019)), around 46% of rabies deaths occur in Asia and around 33% in Africa. This means the majority of the disease burden is located in LMICs[1].
1.3 Rabies treatment
Rabies has a fatality rate of 100% if untreated (WHO, 2023). However, treatment in the form of post-exposure prophylaxis (PEP) is 100% effective if properly administered. It consists of a dose of human rabies immune globulin (HRIG) and a course of several rabies vaccines. People who have been previously vaccinated or have received pre-exposure vaccination usually do not receive HRIG (CDC, 2022). The average cost of PEP is around $108 and can be as high as almost $600 (FAO et al., 2018). This excludes patients’ costs for lost income and travel to health clinics, which can be considerable given that 80% of rabies cases occur in rural areas (FAO et al., 2018). Combined with the fact that most rabies deaths happen in Asia and Africa, it can be assumed that treatment is often inaccessible for the people who need it most.
1.4 Rabies prevention
As stated above, virtually all global human rabies cases are transmitted by dogs (WHO, 2023). One way to reduce the global burden of disease is lowering the cost of and increasing access to PEP (Stokstad, 2017; WHO, 2023). However, a more cost-effective and better long-term solution to fighting dog-mediated rabies could be tackling the problem at the source, which means reducing rabies incidences in dogs directly via dog vaccination (Mindekem et al., 2017).
Since rabies is an infectious disease, the virus needs to be properly controlled in order to prevent outbreaks. The WHO recommends vaccinating at least 70% of the dog population in order to manage canine rabies and keep infections at bay (WHO, 2023). In the best case, high dog vaccination rates could altogether eliminate rabies in certain regions. This has been shown by the Gates Foundation (WHO, n.d.) as well as by the fact that rabies has been widely eliminated in Europe and the Americas (see for example Gibson et al., 2020)[2].
Traditionally, dogs are vaccinated using parenteral (i.e., injectable) vaccines, which are cheap and easy to administer, particularly to homestead dogs (WHO, n.d.). However, reaching a 70% vaccination coverage is not always feasible only using parenteral vaccines, since many stray and free-roaming dogs are difficult to capture and restrain in order to inject them. In addition, dog-catching teams need to receive extensive training, and capturing free-roaming dogs is time-intense as well as dangerous (Yale et al., 2022; Chanachai et al., 2021). This means that a significant portion of free-roaming dogs remains unvaccinated and potentially rabid.
1.5 Oral rabies vaccination
Oral rabies vaccines (ORV) could help close the vaccination gap, since they are better-suited to target stray and free-roaming dogs (WOAH, 2022; Freuling et al., 2023). Instead of injecting the vaccine, oral vaccines can be administered without touching or coming too close to a dog. ORVs usually come in liquid form, contained in a plastic sachet and inside a bait, which the dog will eat. When chewing the bait, the dog perforates the sachet and the vaccine is released into the dog’s mouth, where it is taken up by the palatine tonsils (Yale et al., 2022).
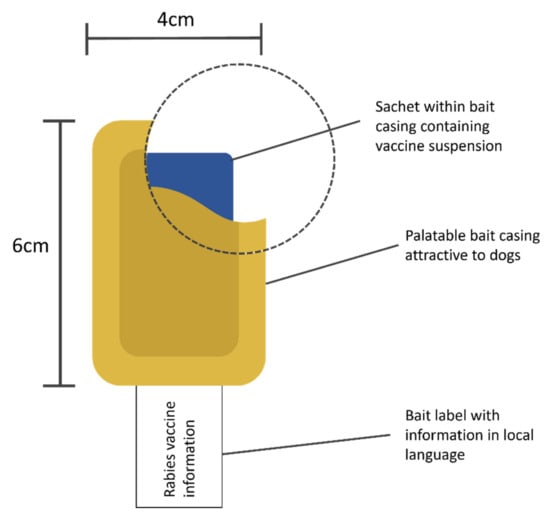
Figure 1: ORV bait (Yale et al., 2022)
The baits come in different variations and can be produced locally (e.g., meatballs, boiled intestines) or manufactured on an industrial level (e.g., IDT Biologika’s egg bait). While different baits appeal to different dog species in different geographies, the WHO (2023) and others (Chanachai et al., 2021; Freuling et al., 2023) recommend using industrially manufactured baits. Oral vaccines require a stable cold chain at freezing temperatures to keep their potency (WHO, 2023), so interrupting the cold chain to put vaccine sachets inside pieces of meat or intestines during local manufacturing (after which the baits need to be re-frozen until they are used) can reduce the vaccine’s effectiveness. When using an industrially manufactured bait (i.e., when buying the vaccine, the sachet is already inside a bait), the vaccine sachets do not need to be taken out of freezers in order to put them inside baits.
ORVs can be distributed amongst dogs in different ways (Cliquet et al., 2018; WHO, 2023):
- Door-to-door model: vaccination teams visit individual homes and either administer ORVs to the dog directly, or hand over the bait to the dog’s owner to administer later. This method only targets owned dogs and requires dog owners to handle the ORV, potentially interrupting the cool chain if the dog is not at home and the bait only offered at a later time. This method can also be very time consuming.
- Wildlife model: baits are placed at selected sites where they are accessible to free-roaming dogs. After 18–24h, baits that have not been eaten are recollected. This method is well-suited for reaching dogs that avoid human contact; however, it bears a greater risk of humans and non-target animals coming into contact with the bait. Since dog rabies vaccines are often live virus vaccines, they bear the risk of infection or mutation.
- Hand-out and retrieve model: vaccination teams distribute baits to each dog they encounter while moving through an area. If a dog does not accept the bait, it is recollected to avoid bringing it into contact with humans or non-target animals. This method is well-suited to target free-roaming owned and ownerless dogs, but not homestead dogs.
Previous mass vaccination campaigns have shown that ORV can be effective in helping to reduce and even eliminate rabies transmitted by certain animal species in certain regions, such as fox-mediated rabies in vast areas of Western and Central Europe (Freuling et al., 2013).
2 Theory of change
2.1 Scope of the intervention
The aim of this intervention is to reduce (and possibly eliminate) canine rabies, and consequently reduce rabies deaths in humans. This intervention only focuses on dog vaccination to reduce canine rabies incidences, not on dog population management (e.g., through sterilization or contraception).
As there are other large health organizations acting in this space (e.g., WHO, Global Alliance, World Organisation for Animal Health (WOAH)), cooperation with other stakeholders is likely to contribute to larger efforts aiming at eliminating dog-mediated rabies. Elimination of rabies could be achieved by combining a range of different strategies, such as information campaigns about dog bite prevention, increasing access to post-exposure prophylaxis (PEP), and dog vaccination (WHO, n.d.). The intervention researched in this report uses only one of these strategies, so I assume it could be integrated into larger rabies elimination campaigns. This means that working closely together with national and international health authorities in the field of rabies elimination could increase the impact of this intervention beyond the immediate reduction of human rabies cases. Cooperation with other actors could also help to coordinate and synchronize vaccination campaigns of this intervention with other vaccination campaigns, e.g., with parenteral vaccination of homestead dogs or campaigns in surrounding rural or urban areas (Yale et al., 2022).
2.2 Causal chain
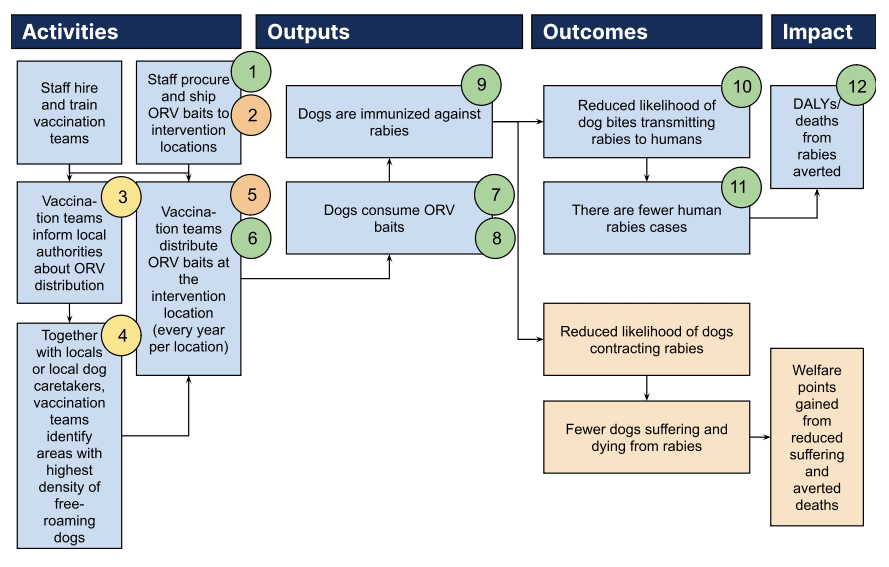
2.2.1 Staff training
The organization hires and trains vaccination teams which will vaccinate dogs. These teams consist of two to four veterinary staff, and will be trained in—amongst other things—bait handling, techniques for approaching dogs, methods of offering vaccine baits, recording vaccine bait handling by each dog, interpreting the effectiveness of the vaccination attempt, and retrieving discarded vaccine sachets after bait consumption.
2.2.2 Bait procurement
The organization (potentially in cooperation with partner organizations) procures industrial manufactured baits in large quantities and ships them to intervention locations. The logistics of this may be challenging since the baits need to be kept frozen throughout shipping and transporting. A few hours before vaccination teams offer the baits to dogs, the baits need to be thawed.
2.2.3 Bait distribution
At each intervention location, one or multiple vaccination teams move through the village, especially around places often frequented by free-roaming dogs, and distribute baits to those dogs they encounter. This distribution is called the “hand-out and retrieve” method and has been used in several other trials of this intervention in the past (see Chanachai et al., 2021; Freuling et al., 2023; Gibson et al., 2019; Smith et al., 2017). To avoid environmental pollution and minimize the risk of humans (especially children) and non-target animals coming into contact with the vaccine, the vaccination teams retrieve and properly dispose of all baits that have not been consumed. To know which dogs have already consumed a bait and prevent re-vaccinating dogs, they can be marked with temporary paint (see Bonwitt et al., 2020).
To ensure that the distributed baits are accepted and consumed by the majority of dogs, a trial to test out the acceptance of different baits might be included in the pilot program before rolling out the intervention on a larger scale.
2.2.4 Cooperation with local communities
Previous to distributing baits in a community, the organization contacts local authorities in the village and informs them about the intervention. Depending on a country’s or region’s legal requirements around oral rabies vaccination distribution, official approval needs to be obtained. Further, official buy-in can be important in order to gain public support for the campaign and to not be met with villagers’ misconceptions (e.g., people believing the baits are poisonous to kill or sterilize dogs).
The organization also seeks contact with locals, especially local caretakers of free-roaming dogs, in order to facilitate locating areas with the highest density of free-roaming dogs and to make the dogs feel more comfortable (and thus more likely to consume the bait) in caretakers’ presence when distributing the baits.
2.2.5 Monitoring
In order to make sure the vaccination was successful, the vaccination team monitors each dog closely while distributing the baits. Dogs need to chew the bait multiple times in order to perforate the sachet including the liquid vaccine, which then gets absorbed via a dog’s oral mucosa. If a dog swallows the bait as a whole, it will likely not get immunized (Yale et al., 2022).
Several studies and reports stress the importance of dog vaccination campaigns of reaching a critical vaccination coverage threshold (~70%) to effectively and permanently reduce canine dog incidences (Zinsstag et al., 2009; Stokstad, 2017; Franka et al., 2013; WHO, 2023). For the organization to assess vaccination coverage in each village, it needs to estimate local dog population numbers as well as monitor vaccination success as described above. As coverage wanes over time (since the vaccine loses its immunity, new dogs are born, or unvaccinated dogs migrate to a given region), the immunization campaign likely needs to be repeated every year.
2.2.6 Intervention result
As a result of the oral rabies vaccination campaigns, canine rabies incidence at the intervention locations is reduced, which leads to fewer dog bites (due to reduced aggressiveness in dogs) and to dog bites bearing a reduced risk of transmitting rabies to humans. Ultimately, the intervention aims to avert premature deaths from rabies.
2.2.7 Potential positive externalities of this intervention
According to WebDM (2023), there is no rabies treatment for dogs, so vaccination is the only way to prevent dogs from suffering and dying from rabies. Rabies symptoms last around a week and include paralysis, seizures, breathing difficulties, swallowing difficulties, aggression and self-mutilation (CDC, 2011), which imply a lot of suffering for a rabid dog in the days leading up to its death.
Vaccination does not only prevent dogs from contracting rabies, they might also protect from other infectious diseases. According to Knobel et al. (2017), rabies vaccination reduced the risk of death in dogs by 56–16% (depending on the dog’s age). The study was done on parenteral vaccination, so the findings might not generalize to oral vaccination.
Lastly, reducing canine rabies cases through dog vaccination might decrease the use of dog culling as a way to reduce rabies incidence rates. In some regions in Africa and Asia, mass dog culling is used as a form of population control to respond to rabies outbreaks. However, this method is generally quite ineffective in controlling rabies (Taylor et al., 2017). I am quite uncertain about this externality, since the effect of dog vaccination on dog culling practices likely relies on people’s attitudes and beliefs around rabies transmission, which I have not looked into.
2.2.8 Potential negative externalities of this intervention
ORVs can pose risks to human and non-target animals if they come into contact with the vaccine. These risks are discussed in more detail in section 3.3.7.
As discussed in the previous section, effectively immunizing dogs against rabies means dogs have a reduced risk of dying from rabies and likely live longer. This has several implications:
- A dog’s longer lifespan in and of itself might be a positive or negative externality depending on whether a dog’s life is net positive or net negative. The intervention would largely target free-roaming (but mostly owned) dogs in LMICs, which means that these dogs are not confined and ideally get fed and maybe even taken care of if they get ill. However, free-roaming also means that they might be exposed to heat or cold and experience injuries.
- Dogs that live longer can reproduce more often, which might increase the total dog population in a region, potentially leading to less available food (and thus reduced welfare) as well as mass dog culling to control the growing dog population.
- Dogs relying on a meat-heavy diet and living longer will cause the suffering and killing of animals used as their food.
- The baits used in oral vaccination campaigns are made of meat, fish or eggs. This means that if the intervention is carried out on a large scale, a significant amount of fish, meat or eggs are required, causing harm to the animals from which these products come.
Developing a rabies vaccine for dogs likely involves animal testing in order to study the effectiveness of the vaccine. Some of the studies conducted might intentionally infect dogs with rabies in order to study the vaccine’s effect, leading to dogs’ suffering and death if the vaccine is ineffective. I have not researched if such studies are actually conducted. Assuming they are, it could be argued that these studies provide enough instrumental value (i.e., developing an effective dog vaccine that further down the line protects dogs from rabies) to justify the direct suffering and death inflicted on the studied dogs.
All of the externalities on dogs are highly uncertain and heavily depend on the probability of dogs’ sentience, moral weight and overall welfare. However, due to the limited time available for this report, going forward I will only focus on the potential benefits for humans gained from this intervention. If this intervention were to be implemented, I strongly recommend further looking into the externalities on animal welfare given that the number of animals reached by this intervention could be high.
2.3 Outlining assumptions and levels of uncertainty
1) Low uncertainty – ORVs and baits are readily available on the market and can be procured in bulk. Since at scale, this intervention would involve vaccinating potentially thousands of dogs, procuring dog rabies vaccines in large quantities is necessary in order to successfully implement vaccination campaigns.
2) Medium-high uncertainty – The necessary cool chain is maintained throughout all of shipping and transportation to the intervention location in order to protect the potency of the vaccine. Most dog rabies vaccines need to be stored and transported at freezing temperatures to maintain their potency (see section 1.5). Given that this intervention is most likely to be implemented in LMICs, robust cold chain infrastructure could pose difficulties.
3) Medium uncertainty – Local authorities are open to cooperating and give their approval for ORV distribution. I assume that vaccination campaigns are more likely to be successful if local authorities are included in their implementation.
4) Medium uncertainty – Locals are open to cooperating and working together with vaccination teams. Similarly to the assumption above, I assume that vaccination campaigns are more successful if locals are involved, for example, to help identify places often frequented by free-roaming dogs and approach dogs.
5) Medium-high uncertainty – ORV campaigns need to be repeated annually. I expect immunity to fade overtime in a given dog population that has been vaccinated, for example due to dog population turnover, dog migration or rabies transmission from wildlife. Thus, vaccination campaigns likely need to be repeated on a regular basis. My first guess is an annual interval.
6) Low uncertainty – Free-roaming dogs pose a significant risk of transmitting rabies. ORV is especially well-suited for targeting dogs that are difficult to approach and vaccinate with a parenteral vaccine. Given that parenteral vaccines are generally less expensive than oral vaccines, this intervention would only make sense if a large proportion of rabies risk comes from free-roaming dogs.
7) Low uncertainty – ORV does not pose risks to dogs, humans and non-target species. ORVs are usually live vaccines and may pose risks of infection or other adverse reactions when used for vaccinating dogs, or when humans or non-target animals accidently come into contact with distributed baits.
8) Low uncertainty – The industrially manufactured egg bait has a high acceptance rate amongst targeted dogs. Given that egg baits are recommended by several studies (see section 1.5), the egg bait needs to be well accepted by dogs in order to be able to vaccinate a large number of dogs.
9) Low uncertainty – The ORV is effective in immunizing the dog against rabies. The intervention would make little sense if the vaccine itself proves to be ineffective.
10) Low uncertainty – The ORV is effective in preventing dog-to-human transmission of rabies. This assumption goes hand in hand with the previous assumption and is fundamental for the intervention to make sense.
11) Low uncertainty – Dog bites cause the majority of human rabies cases and there aren’t any other major ways of transmission of rabies to humans. The effectiveness in the intervention depends on whether other animal species are a major rabies transmitter to humans. If so, only vaccinating dogs would only partly reduce human rabies cases and deaths.
12) Low uncertainty – The people contracting rabies would not have access to PEP. Vaccinating dogs against rabies will only affect human rabies deaths if a substantial proportion of infected people do not have access to PEP.
I discuss all of these uncertainties in more detail in section 3.
3 Quality of evidence
Link to the spreadsheet: Evidence Review: Oral rabies vaccination of dogs
3.1 Evidence that a charity can make a change in this space
3.1.1 What vaccination coverage needs to be reached in order to control and prevent rabies outbreaks?
As laid out in section 1.4, the WHO recommends a vaccination rate of around 70% in order to manage canine rabies and keep infections at bay (WHO, 2023). In section 3.1.2 I evaluate whether ORV campaigns could be successful in reaching a certain vaccination coverage. However, in order to be confident that the 70% vaccination coverage is valid and should be taken as the goal of ORV campaigns, I want to scrutinize this number:
- Coleman & Dye (1996) tests the validity of the recommended 70% vaccination threshold by using epidemic theory together with data from rabies outbreaks in urban and rural areas in the US, Mexico, Malaysia and Indonesia. Their model returns 39–57% as the critical percentage of dogs which need to be vaccinated to prevent or control a rabies outbreak. They report their upper limit to be 55–71%, which is generally below 70%. They conclude that a “coverage of 70% to prevent a major outbreak from occurring on no fewer than 96.5% of occasions”.
- Fitzpatrick et al. (2012) estimate that based on epidemic data from the Serengeti and Ngorongoro districts in Tanzania, the annual vaccination coverage to be achieved is 42–67%, so lower than the recommended 70%.
- Kitala et al. (2002) estimate that based on epidemic data from Kenya, 59% (95% CI 34–70%) of dogs should be vaccinated at any one time. This implies approximately 70% coverage for annual but only 60% coverage for semi-annual vaccination campaigns.
- A systematic review of 19 modeling studies by Rattanavipapong et al. (2018) (which includes Coleman & Dye (1996) and Kitala et al. (2002)) found that assuming annual vaccination campaigns, most studies validated the recommended 70% vaccination coverage.
All in all, the evidence shows that the needed vaccination coverage is dependent on the region a vaccination campaign is carried out, and that for some regions it can be lower than the recommended 70%. The 70% coverage seems to be an upper bound to control and prevent rabies outbreaks in most cases[3]. However, given that I do not know the epidemic parameters of the regions where this intervention would be implemented, I will use 70% as the threshold to assess further evidence on the effectiveness of ORV campaigns.
3.1.2 What vaccination coverage do ORV campaigns reach?
Overall, there is limited evidence available on the effectiveness of ORV campaigns reaching the 70% vaccination threshold. The three most relevant studies that I could find are as follows:
- Chanachai et al. (2021) used egg baits and a hand-out and retrieve model, which make their intervention very similar to the one researched in this report. They estimate a 65.6% vaccination coverage[4] of all dogs they identified (average across five regions). The coverage of the total dog population is probably lower, since I assume they did not identify the whole dog population.
- Freuling et al. (2023) also used egg baits and a hand-out and retrieve method and reported that 83% of dogs who were offered a bait were considered vaccinated. As is the case above, the coverage of the total dog population is probably lower, since I assume they did not offer baits to the whole dog population at the intervention location.
- Undurraga et al. (2020) modeled that their ORV campaign combined with parenteral door-to-door vaccination reached a vaccination rate of 78.3% (95% CI 77.0–80.0%) of the total dog population and 53.1% (95% CI 46.6–59.6%) of the free-roaming dog population. The gap in coverage between the free-roaming and total dog population might be explained by two details of the study: the vaccination teams defaulted to using parenteral vaccines and only used ORVs if a dog was not approachable; and the vaccination team ran out of ORV baits during the campaign. The results are of further limited generalizability since the campaign did not solely use ORV vaccines.
Overall, the studies reviewed above provide only weak evidence, either because they did not estimate vaccination coverage for the whole dog population (only for identified dogs) or their campaign used parenteral vaccines in addition to oral vaccines. Further, the studies above were conducted in Thailand, Namibia and Haiti, respectively. It is unclear how the results generalize to a different country where conditions (e.g., dog population density, proportion of free-roaming dogs) may differ.
Furthermore, none of the above studies report a baseline vaccination rate for the region where they conducted the study. I assume that the higher the baseline coverage, the likelier it would be to reach the 70% vaccination threshold (i.e., if the baseline coverage was 10% instead of zero, reaching the 70% threshold would be more likely).
In light of the evidence presented in this section, my rough and quite intuitive estimation is that it is 30–80% likely that an ORV campaign can reach a 70% vaccination coverage of the total dog population at a given intervention location. The upper and lower bound should reflect that I expect that the 70% vaccination coverage could be quite difficult to reach but, as outlined above, might be more likely with a higher baseline coverage. One caveat to mention is that while a single campaign might not reach the 70% threshold, several subsequent campaigns might be, for example in one-year intervals. Immunity seems to last longer than one year, and subsequent campaigns could reach dogs that were missed in previous campaigns, which would increase the overall vaccination coverage.
The evidence review in this section is not exhaustive and I expect that given more time, additional studies could be found that could update my estimated probability range.
3.2 Evidence that the change has the expected effects
3.2.1 How effective are ORV campaigns at reducing the incidence of dog-mediated rabies in humans?
Cleaveland et al. (2003) use a difference-in-difference experimental design to assess dog rabies and human dog-bite incidence rates in two districts in rural Tanzania, with one district getting four rabies vaccine campaigns (conducted in roughly 1-year intervals) and the other one acting as the control area. Each vaccination campaign in the intervention area reached a vaccination coverage of 61.1–70.6%. In the intervention area, the dog rabies incidence rates decreased by 64.9–93.8% (p<0.001) and the human rabies incidence rates decreased by 51.0–92.0% (p<0.001) after each campaign, compared to the baseline yearly incidences of 0.00146% (1 in 685 dogs) and 0.000288% (1 in 3470 people), respectively. In the same period in the control area, the canine dog incidence decreased by 17.2–37.1% (p=0.22) and the human dog incidence increased by 138.5–163.3% (p=0.06), compared to the baseline of 0.01089% (1 in 91 dogs) and 0.000117% (1 in 8550 people), respectively. This study suggests vaccination campaigns have quite a large effect on dog rabies and human dog-bite incidence rates. However, the evidence is of limited strength, since the experimental design is not randomized, which means that the two districts could be systematically different from each other and the changes in incidence might not be entirely attributed to vaccination. The study further loses strength in failing to mention the exact sample size (it can be assumed that it includes >20 villages).
Zinsstag et al. (2017) report canine and human rabies incidence rates after two vaccination campaigns (with a one-year interval in between) in one city in Chad. Both campaigns reached around 71% of dogs. While the baseline for the canine rabies incidence rate was 0.000033% (1 in 30,000 dogs) per week and the baseline for human rabies exposure rate 0.000001% (1 in 1,000,000 people) per week, the endline rate was 0.0000016% (1 in 635,000 dogs) and 0.000000002% (1 in 500,000,000 people), respectively, which mark a respective 95.2% and 99.8% decrease. While this study also suggests a very large effect of vaccination campaigns on incidences, the evidence is quite weak (weaker than Cleaveland et al. (2003)) since the experimental design was a simple pre-post comparison without a control group and had a sample size of n=1.
Notedly, the two studies are based on parenteral vaccination campaigns, not on ORV campaigns. However, I expect this to matter little if an ORV campaign reaches a similar vaccination coverage as the studies above do. Also, neither of the two studies looks at human rabies incidence directly, only at dog rabies and human dog-bite incidence. Not every bite from a rabid dog leads to rabies (Di Quinzio & McCarthy, 2008), so the exact effect of vaccination on human rabies incidence and deaths remains unclear. It also has to be noted that while the effect observed in both studies is quite large, the baseline and endline incidence rates are quite low, which could reduce the cost-effectiveness of this intervention.
My evidence review for this section is not exhaustive and I expect that if I spent more time on it, I could find more studies on the effect of ORV campaigns on human rabies incidence. However, given that within the time I spent on this section I was only able to find two studies and no RCTs leads me to conclude that the overall evidence base on the effect of ORV campaigns is quite weak.
Overall, I base more weight on the study by Cleaveland et al. (2003) due to its more robust experimental design. I discount their findings by 10% based on the fact that the study was not randomized and that it is unclear how generalizable the results are to other regions or countries. Thus, I estimate that annual vaccination campaigns (which reach the 70% vaccination coverage) can decrease canine rabies incidence rates by 51–61% and human dog-bite incidence rates by 55–82%.
A note on consideration around implementing this intervention: I expect monitoring dog and human rabies cases to be a major challenge requiring a robust reporting and surveillance infrastructure for regions and countries where there is not already one in place. Given that not all people with potential rabies exposure seek treatment (see section 3.2.3), monitoring rabies cases is likely not possible in a centralized (i.e., on the level of health facilities) manner without missing many potential rabies cases. This means that establishing a robust feedback mechanism for this intervention might be a far bigger challenge than I previously anticipated (and indicated in the theory of change).
3.2.2 What is the effect of reducing human rabies cases on the number of human rabies deaths?
Reducing the number of human rabies cases does not necessarily lead to reducing human rabies deaths, since many or even all of the cases could be effectively treated with PEP (since PEP is almost 100% effective if administered properly and in time (FAO et al., 2018)). Thus, in this section I want to assess what percentage of people do have access to PEP and whose death could counterfactually be averted by preventing them from contracting rabies in the first place:
- Changalucha et al. (2019) find that across different rural and urban settings in Tanzania, 25% of people with probable rabies exposure did not seek PEP. Of those who did seek care, 15% did not receive PEP due to shortages, cost barriers or misadvice. Of those who received PEP, 46% did not complete the course. This means that of all people potentially exposed to rabies, only 34% of people received adequate PEP.
- Sreenivasan et al. (2019) find that the availability of post-exposure vaccination was limited in 7 out of the 22 (32%) African and Asian countries they assessed. No information on availability of immunoglobulin (which also forms part of PEP) is reported.
- Knobel et al. (2005) use an epidemiological model and estimate the number of rabies deaths in Africa and Asia to be 55,270 and predict the hypothetical number of deaths in the absence of any PEP to be 327,160. According to these numbers, 14% of people with rabies are estimated to not receive adequate PEP. While this study seems to be very comprehensive compared to the other studies, it is also almost 20 years old, so I put less weight on it. Furthermore, I expect PEP accessibility to vary widely from the reported average of 14% on a per-country basis.
PEP accessibility is not the only relevant factor but also how willing people are to seek it out based on how much they know about the fatality of rabies and the existence of PEP. For example, Masthi et al. (2019) found that in India, only 60.4% of participants (68.9% in urban and 56.5% in rural areas) had heard of rabies. Of those that knew about rabies, 72% (64.9% in urban and 76.1% in rural areas) were aware that it was a fatal disease. Upon rabies exposure, 63.5% would seek care at a medical facility, 22.9% would only wash the wound, 5.7% would apply traditional medicines and 9.5% would do nothing.
Given that I expect PEP accessibility and knowledge and practices around rabies to vary between regions and countries, it is difficult to conclude a general percentage of people whose life could be saved through preventing them from getting rabies in the first place. Changalucha et al. (2019) and Masthi et al. (2019) provide the most detailed data, but their findings are limited to one specific country each. As a rough estimate, I thus think on average 25–45% of people with rabies do not receive adequate PEP. This is somehow close to the proportion observed by the two studies but with a wider range due to my high uncertainty. However, depending on the exact intervention location, the number could be much higher. People in rural areas might have more difficulties accessing PEP so if ORV campaigns were conducted in these areas, it could be possible to avert more deaths, everything else being equal.
As in the previous section, the evidence review in this section is not exhaustive and I am quite certain that given more time, additional studies could be found that would update my estimation.
3.3 Evidence on other key uncertainties
Due to time constraints, I spent the majority of the time for this section on the uncertainties marked as orange and yellow in section 2.3. For assumptions marked as green, only a limited amount of literature was reviewed, and reviewed superficially.
3.3.1 ORVs and baits are readily available on the market and can be procured in bulk
Unfortunately, I did not find any direct information on the mass availability of ORVs. What makes me positively update is the fact that the global oral rabies vaccine market is predicted to grow from $80 million in 2022 to $297 million in 2029 (QYR Research, 2023). Currently, there seem to be three big ORV producers—Boehringer Ingelheim, Virbac, Ceva Santé Animale—and one large egg-bait producer—IDT Biologika. ORVs are also currently used in mass vaccination campaigns, for example a racoon vaccination campaign in the US using 365,000 baits (USDA, 2023). In the past, they were used in mass vaccination campaigns for foxes in Europe (Freuling et al., 2013). One piece of information that makes me slightly negatively update is the WOAH (2022) reporting that currently only limited ORVs are produced due to less demand. However, it is unclear what absolute number of ORVs falls under “limited”. Overall, I think the positive evidence weighs more heavily than the negative one, which is why I am quite certain of this assumption.
3.3.2 The necessary cold chain is maintained throughout all of shipping and transportation to the intervention location in order to protect the potency of the vaccine
Many oral rabies vaccines, including the egg bait recommended for ORV campaigns, have the big disadvantage of needing to be stored at deep freezing temperatures (at around -18 to -20°C) until they can be thawed and kept in a cooler until their field use (Freuling et al., 2022; Chanachai et al., 2021). This makes the logistics of transporting ORVs from the producer to the intervention location much more difficult since vaccines need to be part of a robust cold chain, otherwise they lose their potency (WHO, 2023). These cooling requirements make me highly uncertain about recommending this type of bait after all, since there seem to be other vaccines which only need to be kept at cooling temperatures of 4–8°C (see Smith et al., 2017). However, the choice of which vaccine to use is not only dependent on storage requirements but also on its acceptance by dogs, vaccine release and time needed for manufacturing baits (if industrially manufactured baits are not used). So in this section I will still assume that the intervention will use egg baits, and look into cold chain logistics required for transporting them.
In general, cold chains seem to be a substantial problem in LMICs. For example, Kasahun et al. (2023) find in their meta analysis that “the overall/pooled prevalence of good vaccine cold chain management practice in Ethiopia is 27.48% with 95% CI (25.70–29.26).” Burstein et al. (2013) found that in Ghana, Kenya and Uganda, around 17% of storage facilities stored vaccines outside of recommended temperatures. The WHO (2017) reports that dysfunction cold chains seem to be an issue for vaccine distribution in general.
What makes me slightly more certain of this assumption holding is the fact that both Chanachai et al. (2021) and Freuling et al. (2022) used the egg baits and seemed to have successfully managed the cold chain logistics. Nevertheless, their studies took place at a much smaller scale than an organization implementing this intervention potentially would.
Based on the cold chain management by Chanachai et al. (2021) and Freuling et al. (2022), I imagine transportation could look like the following: ORVs could be shipped on dry ice from the producers to a large city (which is more likely to have stable electricity and freezers) in the operating country. Up until then, already existing cold chain logistics could be used. After that, portable cold boxes filled with dry ice could be used to transport vaccines to the intervention location. Dry ice can last around 24–48h if cool boxes are properly sealed off (Linde Gas & Equipment Inc., n.d.) and I expect transportation from the central storage to the intervention location will not take more than two days. At the intervention location, the ORVs can already start thawing since they generally last around three days when refrigerated. This would eliminate the need for freezers at the intervention location and only require refrigerators. Vaccination teams could take refrigerators with them to the intervention location (this would require stable electricity at the location), or use available refrigerators or ice packs at primary health care centers. As vaccines should not be thawed and refrozen, vaccination teams need to make sure to only take as many vaccines with them as they need, since the vaccines they will not use will likely perish. One potential way to reduce the risk of exposing vaccines to high temperatures if the cold chain fails is planning vaccination campaigns for cooler months of the year (Yale et al., 2022), although this could pose a limitation when aligning ORV campaigns with other rabies vaccination campaigns.
All in all, I remain highly uncertain of this assumption, since a large part of the cold chain logistics remain outside the control of the organization (e.g., transportation to central storage, electricity access). The development of a vaccine that does not need to be frozen (or ideally cooled) would be a game changer, and it seems like initial efforts to do this are already made (Smith et al., 2015).
3.3.3 Local authorities are open to cooperating and give their approval for ORV distribution
A range of trials of this intervention have already been conducted (see Kasemsuwan et al., 2018; Husein et al., 2023; Chanachai et al., 2021; Bender et al., 2017; Gibson et al., 2019), which is weak but positive evidence that authorities will be willing to cooperate. Paired with the general positive public attitudes towards rabies vaccination (see section 3.3.4), I am somehow certain this assumption would hold, but given the limited time I spent researching this assumption, I will keep it marked as “medium uncertainty”.
3.3.4 Locals are open to cooperating and working together with vaccination teams
Studies on people’s attitude towards rabies vaccination is positive evidence that this assumption would hold:
- Iddi et al. (2023) found that 90% of survey people in Tanzania believed it was necessary to vaccinate all owned dogs.
- Ung et al. (2021) found that 97% of surveyed people in Cambodia were willing to vaccinate their dog against rabies if the vaccination was provided for free.
- Freuling et al. (2022) found that 99% of dog owners they approached in Namibia agreed to have their dogs vaccinated using ORV.
However, it might be the case that people have more positive attitudes toward dog vaccination in regions with already higher vaccination rates.
Chanachai et al. (2021) successfully worked together with local municipality workers and dog caretakers to identify the places free-roaming dogs are usually found, as well as to help approach these dogs. If these people do not cooperate, I suspect it will be possible to identify popular places of free-roaming dogs without their help, and studies could be used to identify common places where dogs can be found (see Silva et al., 2022; Raynor et al., 2020).
Nevertheless, given the limited time I spent researching this assumption, I will mark it as “medium uncertainty”.
3.3.5 ORV campaigns need to be repeated annually
Reaching a vaccination coverage of 70% in a given dog population might only be reached with several subsequent vaccination campaigns. Determining the interval between these campaigns likely depends on multiple factors, including the duration of immunization effects, dog population growth, and dog movement/migration.
Regarding the duration of immunity, Freuling et al. (2019) finds that the immunity of ORVs lasts for at least one year. Studies on parenteral vaccines show that their immunity lasts for at least two (Zhang et al., 2007) and maybe even up to six years (Dodds et al., 2020), however I am unsure how generalizable these findings are to ORVs.
Annual campaigns[5] seem to be widely established or recommended when looking at the available literature (see Hampson et al., 2009; Townsend et al., 2013; Rattanavipapong et al., 2018; Conan et al., 2015; Moore et al., 2017). However, many studies note that the exact timing depends on the vaccination coverage reached by a campaign, the birth rate of a given dog population (seen newly born dogs are not vaccinated and reduce the vaccination coverage), and the duration of immunity of the used vaccine.
Overall, I remain very uncertain that the vaccination interval should be one year. Although many trials use this interval, there seem to be too many factors that could influence this interval. Implementers of this intervention should have a closer look at these factors in order to set a suitable campaign interval.
3.3.6 Free-roaming dogs pose a significant risk of transmitting rabies
ORVs are especially well-suited to reach dogs that generally cannot be reached with parenteral vaccines. As ORVs are generally more expensive than parenteral vaccines (Undurraga et al., 2020), using ORVs for dogs that could be vaccinated parenterally would be unnecessarily costly. By contrast, using parenteral vaccines and catch-vaccinate-release models for free-roaming dogs usually has higher personnel and equipment costs for targeting free-roaming dogs . So in order for ORV campaigns to have a substantial effect on vaccination coverage and remain cost-effective, a large proportion of the total dog population in a targeted region need to be free-roaming (Cuddington & McAuliffe et al., 2023):
- Menghistu et al. (2018) found that in the studied communities in Ethiopia and Kenya, around 60% of dog owners did not control their dogs during the day, which means they were free-roaming during this time.
- Warembourg et al. (2021) found that owned[6] free-roaming dogs are the main vector of rabies transmission to humans worldwide.
- Ngugi et al. (2018) found that 78% of dog bites in Kenya are from owned free-roaming dogs.
- The WOAH (2022) states that free-roaming dogs are a major reservoir of rabies virus in Asia.
Overall, this evidence makes me quite certain that free-roaming dogs are responsible for a large proportion of rabies transmission. Thus, when implementing this intervention, regions with large proportions of free-roaming dogs should be prioritized.
3.3.7 ORV does not pose risks to dogs, humans and non-target animals
The WHO (2023) states that ORVs are usually live vaccines and may pose risks of infection or other adverse reaction when humans or non-target animals accidently come into contact with distributed baits.
As far as my research revealed, the risks posed by ORV are very low:
- The WOAH (2022) states that the results of a simulation study of ORV distribution in India resulted in no human deaths for 10 million distributed baits and 0.3 dog deaths for 1 billion distributed baits
- Smith et al. (2017) reported no incidences of human exposure or dog deaths during their trial of 590 distributed baits.
- Wallace et al. (2020) states that only very sporadic adverse events in humans were reported across several ORV campaigns.
- Cliquet et al. (2018) state that during an ORV campaign in Finland including 360,000 baits, nine dog owners reported adverse reactions in their dogs, many from ingesting the plastic sachet or from consuming multiple baits.
- Several studies and large-scale wildlife campaigns show the safety of various different oral rabies vaccines for non-target animals (Maki et al., 2017; Mähl et al., 2014; Vos et al., 1999; WOAH, 2022).
Additionally, the hand-out and retrieve model suggested for this intervention seems to be well-suited for minimizing contact with humans and non-target animals (see Smith et al. 2017; Wallace et al., 2020). Altogether, I am quite certain ORV campaigns do not pose any serious risks to dogs, humans or non-target animals.
3.3.8 The industrially manufactured egg bait has a high acceptance rate amongst targeted dogs
ORV can be used with different kinds of baits (e.g., boiled intestines, fish meal, egg), and the acceptance of these baits by dogs target in a given region can vary. The proposed bait for this intervention is an egg bait, and its acceptance has been looked at in multiple studies:
Source | Egg bait | Intestine bait | Fish bait | Gravy bait |
| Kasemsuwan et al., 2018 | 78.77% | 79.19% | 50.29% | - |
| Husein et al., 2023 | 81.1%* | 96.9% | - | - |
| Bender et al., 2017 | 77.4% | 91.9% | 81.1% | - |
| Gibson et al., 2019 | 77.5%* | 68.7% | ||
| Chanachai et al., 2021 | 87.3%* | 92.9% | - | - |
| Bonwitt et al., 2020 | 60% | 86% | 14% | - |
* egg bait was covered with dog/cat food paste
While intestine baits seem to have generally higher acceptance, all except one of the above studies report that egg baits are superior to intestine baits when it comes to vaccine release.
Overall, I am quite certain egg baits will have a high acceptance rate amongst dogs. This could also be easily tested in a pilot program. However, as seen in section 3.3.2, the necessary cold chain logistics for the egg bait could pose a problem, and the intervention might be more easily implementable with locally produced intestine baits if this means more thermostable vaccines could be used.
3.3.9 The ORV is effective in immunizing dogs against rabies
All in all, I found two studies assessing dogs’ antibody levels:
- Smith et al. (2017) found that after a dog vaccination campaign in Haiti, 59.3–77.8% of dogs which consumed an oral vaccine showed antibodies, compared to 92.7% of dogs who received a parenteral vaccine.
- Molini et al. (2021) found that after an ORV campaign in Namibia, 52.9–78.8% of dogs who consumed an oral vaccine showed antibodies (while 10% of dogs already showed antibodies prior to vaccination).
The wide ranges are due to measuring antibody levels in two different ways (i.e., ELISA and RFFIT), which I did not have time to look into. Interestingly, the interviewed experts (see section 5) noted that the 70% vaccination coverage already accounts for not all vaccines distributed to dogs being effective, so the coverage is based on the number of dogs consuming and chewing the bait, instead of the number of dogs that show antibodies.
I weigh both of these results equally strong, since Smith et al. (2017) included a larger sample size and Molini et al. (2021) used egg baits, the kind of baits which are considered to be used in the intervention of this report (Smith et al. (2017) used intestine baits).
3.3.10 The ORV is effective in preventing dog-to-human transmission of rabies
This assumption is discussed in section 3.2.1.
3.3.11 Dog bites cause the majority of human rabies cases and there aren’t any other major ways of transmission of rabies to humans.
The WHO (2023) states that virtually all human rabies cases stem from dog bites. While rabies can be theoretically transmitted from an infected person, no such case has been documented (CDC, 2019).
3.3.12 The people contracting rabies would not have access to PEP
This assumption is discussed in section 3.2.2.
4 Expert views
Dr Thomas Müller and Dr Conrad Freuling
(World Health Organization Collaborating Centre for Rabies Surveillance and Research; World Organisation for Animal Health Reference Laboratory for Rabies; Institute of Molecular Virology and Cell Biology, Friedrich-Loeffler-Institut)
The main insight Dr Müller and Dr Freuling gave me was that a small- to medium-scale ORV campaign will not be effective in reducing rabies long-term:
- Even if a vaccination campaign achieves a high vaccination coverage in a given region (and effectively reduces the number of human rabies cases), the region needs a system of constant rabies surveillance and strict border control when introducing dogs or other animals from outside (like they are implemented in rabies-free regions in Europe, for example). In the absence of these monitoring and control mechanisms, the canine rabies rate will increase again fast.
- Vaccination campaigns need to be rolled out over a large area—ideally a whole country or even across multiple countries—and over a short time period (one or more weeks as opposed to a year) in order to increase the vaccination coverage in the whole region all at once. Doing so requires close cooperation of regional, national, and ideally international authorities, a regional/national/international strategy and both substantial financial resources as well as surveillance infrastructure to tackle the problem at a large scale. One prime example of a large-scale rabies control effort is Latin America, where national governments drove rabies control programs in their respective countries and closely cooperated with other governments in Latin America as well as the Pan American Health Organisation (PAHO), permanently reducing rabies cases.
- While smaller efforts (for example by Vétérinaires Sans Frontières) can reduce local infection sources, these changes will only be temporary, especially without a necessary follow-up strategy.
- They stated that many countries in Africa and Southeast Asia—where rabies control is still very neglected—seem unwilling or incapable of investing in rabies control efforts. Although the WOAH, FAO and WHO provide tools for governments to work out national rabies control strategies, uptake of these tools is low. Large-scale vaccination campaigns are relatively expensive and there is a lack of funding in this space (or countries are unwilling to allocate money) since rabies causes fewer deaths than other infectious diseases such as malaria and tuberculosis. Dr Müller and Dr Freuling pointed out that this is an area where a charity could make an impact: convincing governments to invest in, build the infrastructure for and develop strategies around rabies control.
Apart from these high-level insights, the expert interview corrected some concrete findings of my research and filled some gaps in my report:
- The 70% vaccination coverage already accounts for dog population turnover, and it is not based on the number of dogs who show antibodies after vaccinating, but on the number of dogs who chew the ORV bait and thus are considered vaccinated.
- Rabies not only causes human and animal deaths but also results in people’s financial loss since many farm animals get bitten by dogs and die of rabies, depriving many families of one of their main sources of income.
- Reported data in the field of rabies is very poor due to lack of proper surveillance and reporting infrastructure. Actual rabies deaths might be 20 and 160 times higher than official numbers reported in Asia and Africa, respectively.
- Countries and regions in Southeast Asia that already have a robust national rabies strategy include Thailand and Goa (India), as well as Vietnam, Cambodia and Iran, which have heavily invested in PEP accessibility. In Africa, Tanzania seems to be one of the countries with the most progress, and some efforts are made in Namibia.
5 Geographic assessment
Link to the spreadsheet: Geographic Assessment: Oral rabies vaccination of dogs
5.1 Geographic assessment
I developed a geographic weight factor model (GWFM) to identify countries in which this intervention could be implemented at scale as well as be tractable.
The model uses a lot of proxy indicators since no country-level data was available for many factors I wanted to include. This includes data on (free-roaming) dog populations, PEP accessibility, rabies vaccination coverage in dogs, human rabies incidence, human dog-bite incidence. In some cases, this data might exist spread across multiple sources and studies, so collating this data would have been time intensive and falls outside the scope of this report.
5.1.1 Scale (45%)
Population (40%): Population shows how many people would benefit from a reduced canine rabies and dog-bite incidence. It also serves as a proxy for the dog population of each country. This assumes an equal human-to-dog ratio in each country, which is likely not the case but unfortunately I was unable to obtain this ratio for each country. Dog population shows how many dogs could be potentially targeted by the intervention.
Rabies death rate (60%): The rabies death rate per 10,000 capita is based on Global Burden of Disease data and includes deaths of any age and of both sexes. It is probably the most important factor on the model, since it directly shows the burden of disease from rabies in each country[7]. The rabies death rate does not only indicate the scale of the problem, it also shows its neglectedness. Rabies has been eliminated using mass vaccination campaigns in many countries. In addition, in many countries access to PEP prevents almost all exposed people from dying. This means that countries with a higher rabies death rate probably have a higher canine rabies incidence (i.e., more people get bitten by rabid dogs) and/or do not receive adequate treatment. The latter aspect is included in the neglectedness section.
5.1.2 Tractability (35%)
Logistics Performance Index (25%): As discussed in section 3.3.2, transporting oral rabies vaccines and maintaining the necessary cold chain might pose significant difficulties. The Logistics Performance Index combines country information on customs, infrastructure, international shipments, logistics quality and competence, tracking and tracing, and timeliness (World Bank, 2023), and should broadly capture the general logistics capacity of each country.
Electricity Access (25%): I am uncertain whether the Logistics Performance Indicator captures information on cold chains performance specifically. Thus, electricity access is added to serve as a proxy to indicate how stable cold chains are in each country, since freezers and refrigerators require electricity. The dataset defines electricity access as the share of population with access to electricity and is probably an overestimation of the available electricity for cold chains, since it it is defined as having an electricity source that can provide very basic lighting, charge a phone, or power a radio for four hours a day (Our World in Data, 2020). Vaccines, however, need to be cooled or frozen continuously until they are used, and freezers and refrigerators require more electric power than basic lighting.
Fragile State Index (25%): The Fragile State Index combined information on social cohesion (e.g., security apparatus), economy (e.g., economic development), politics (e.g., state legitimacy) and society (e.g., demographic pressures) (Fragile State Index, 2023) and serves as a marker for general ease of operationality in a country.
Robust national rabies surveillance (25%): Monitoring and evaluating the impact of ORV campaigns is probably much easier if data is available about rabies outbreaks and cases. I assume it would be very costly (both in terms of time and money) for an organization to collect this data itself, so relying on an already existing rabies surveillance system could make the intervention more cost-effective and provide an additional feedback mechanism. The CDC (n.d.) considers a country’s national rabies surveillance as robust “if formal surveillance reports (including methodologies and results) are available in the form of publications, government reports, or other submissions satisfying international reporting requirements”.
5.1.3 Neglectedness (20%)
Universal Health Coverage & Service Coverage Index (25%): Reducing rabies deaths can be tackled from two sides: treating rabies cases or preventing them. While countries with a high rabies death rate probably have a high canine rabies incidence, rabies treatment is likely also not available or else not so many people would die. This means that preventing rabies cases in countries with worse access to treatment can counterfactually avert more deaths. Several studies include data on the availability and cost for PEP, although data for different countries is scattered across multiple studies and is not available for every country. Due to time constraints, I was not able to collate data from different sources and instead used the Universal Health Coverage & Service Coverage Index as a proxy for PEP accessibility. It is based on “the average coverage of essential services including reproductive, maternal, newborn and child health, infectious diseases, non-communicable diseases and service capacity and access“ (OWID, 2022). While some of this information is irrelevant for rabies (e.g., maternal, newborn and child health), it is the information on infectious diseases which is why I included the index in the model.
Global Multidimensional Poverty Index (25%): The Global Multidimensional Poverty Index combines information on health, education and standard of living (UNDP, 2023). According to Taylor et al. (2013), the GMPI is highly correlated with PEP accessibility, which is why I included it in addition to the Universal Health Coverage & Service Coverage Index.
National rabies control program (50%): If a country already has a robust rabies control program in place, it means an organization might have less marginal impact in this country. According to the CDC (n.d.), “a robust national canine rabies control program is evidenced by control measures (such as dog rabies vaccination coverage), significant reduction in cases, and/or transmission limited to focal areas as documented in publications or reports in the past 5 years”.
Existing organizations are not included in the model, since conducting research on all countries would go beyond the scope of this report. Instead, I will look at existing organizations in the top 10 countries according to the GWFM in the next section.
5.2 Where existing organizations work
None of the top ten countries have a national rabies control program (CDC, n.d.). In terms of other actors in this space, Paracon is active in Ghana, Ethiopia, Niger, Mali and Nigeria. However, Paracon does not conduct rabies vaccination campaigns itself but supports governments and other stakeholders to plan and conduct rabies control programs in general. As far as India is concerned, Mission Rabies is operating in Goa. However, they also do not seem to be conducting ORV campaigns but they conduct and provide research to help inform national rabies strategy. In addition, there are several international actors, including United Against Rabies, the Global Alliance for Rabies Control, and the WHO.
Based on the above findings, there seem to be no organizations exclusively dedicated to oral vaccination of dogs in the identified priority countries. I only spent around 1.5 hours researching existing organizations, so it is quite likely I overlooked some.
6 Cost-effectiveness analysis
Link to the spreadsheet: Cost-effectiveness Analysis: Oral rabies vaccination of dogs
My cost-effectiveness analysis models a hypothetical 5-year program in India, where each targeted village would receive three vaccination campaigns in one-year intervals.
The estimated cost-effectiveness of the modeled intervention is $54,268 per averted DALY. According to my model, this intervention would not pass the bar of cost-effectiveness of ~$100 per averted DALY. Moreover, the extent of the impact of this intervention seems to be very limited, with 0.5 rabies deaths averted in total. Notably, the upper and lower bounds of the cost-effectiveness estimate are very wide (I address this issue in section 6.3).
6.1 Effects
I modeled the effects of a rabies vaccination program as follows:
Reach: The reach of the intervention is based on the number of vaccination teams of the organization, the human and dog population per village in which a vaccination campaign is conducted, and the time needed to conduct a vaccination campaign in a given village. This results in an estimate in how many villages an ORV campaign could be conducted, how many dogs would be vaccinated and how many humans could experience the effects.
Intervention effect: The most reliable study I found which looked at the effect of rabies vaccination on rabies incidence provided results for the change in dog rabies incidence and human dog-bite incidence, as opposed to looking at human rabies cases or human rabies deaths directly (see Cleaveland et al., 2003). The study looks at the effects of a three-year vaccination campaign (with vaccines being delivered roughly every year), which is why my modeled intervention also looks at a three-year campaign. The follow-up period of this study is three years; however, I try to model the effects over 10 years, which is why after year 4, the intervention effect is based on my estimation rather on the study.
Baseline dog rabies and dog-bite incidence: The baseline dog rabies incidence is based on Gill et al. (2019) and the baseline dog-bite incidence is cited from John et al. (2022). According to the interviewed experts (see section 5), rabies incidence rates are often an underestimate due to underreporting, which is why I increase the cited numbers by 50–200%.
Rabies transmission risk: Not all bites from rabid dogs lead to rabies infection. Unfortunately, I only found one study (see Di Quinzio & McCarthy, 2008) looking at rabies transmission risk.
PEP accessibility: In order to account for humans with rabies who would get effectively treated (and therefore not benefit from rabies prevention), I used my estimation of the proportion of people having access to PEP from section 3.2.2. Given that I assume the intervention would be implemented in a region with particularly low accessibility, I substantially increased the upper bound.
Discounts: The study by Cleaveland et al. (2003) took place over 20 years ago in Tanzania, which is why I apply a relatively high generalizability discount. I also apply a certainty discount based on the fact that not only the data of the intervention effect but also for other factors is not very robust. Finally, an “other-actors” discount is applied to account for the impact of other actors who might work on rabies control measures in the hypothetical intervention region.
DALY equivalents per premature death: I use the moral weights by Founder’s Pledge in combination with the rough age distribution of rabies victims in order to get to an average number of lost DALYs per premature death caused by rabies.
Overall, the effect of the intervention results from comparing what would happen in the hypothetical intervention region to the counterfactual scenario (i.e., in the absence of the intervention). This means that with the factors outlined above, I calculate the number of rabies deaths that would occur in the intervention region and the number of rabies deaths in the absence of the program. I then subtract one from the other to obtain the number of averted rabies deaths as a result of the program.
6.2 Costs
Start-up charity costs: For better comparability, start-up costs are held constant over all reports.
Ongoing charity costs: Ongoing charity costs include salaries for five staff members over five years, plus overhead costs.
Costs per vaccinated dog: Instead of modeling vaccination costs myself, I relied on the model by Cuddington & McAuliffe, 2023, which estimated costs of $2.90–7.90 per vaccinated dog for campaigns in India. These estimates already include salaries for the vaccination teams, vaccination baits, and vehicle and fuel costs for vaccine delivery. However, they do not seem to include costs for cooling equipment, protection equipment, time spent assessing the achieved vaccination coverage in each village and any unexpected costs, which is why I apply a substantial overhead to the cited costs.
Overall, the costs of the program are the sum of start-up and ongoing intervention costs as well as the cost per vaccinated dog multiplied by the number of dogs being vaccinated according to the expected reach of the program (see section 6.2).
6.3 Limitations
6.3.1 Excluded factors
There are several factors that could lead to the CEA model being an underestimate or overestimate of the real cost-effectiveness:
- The economic losses due to rabies are not included in my CEA, which could mean that the modeled cost-effectiveness is an underestimate. Hampson et al. (2015) estimate that every year in India, $9,050 are lost due to rabies deaths of livestock, and $491,230 are spent on PEP (excluding $42,600 for travel costs and $138,030 for lost income due to seeking treatment). However, it is unclear whether the costs for treatment (plus associated costs) would really be saved as a result of this intervention, since dog rabies needs to be virtually eliminated before a reduction in demand in PEPE becomes apparent (Cleaveland et al., 2003).
- In my CEA, the DALY burden relies entirely on years of life lost (YLL). This is backed up by Hampson et al. (2015) who notes that >99% of the burden of disease of rabies stems from premature death. However, it could be the case that the psychological impact on bite victims and their families are larger than previously assumed. For example, Kaare (2007) finds that 87% of households with dog-bite victims in rural Tanzania feared rabies more than malaria due to unaffordability and unavailability of effective treatment.
- Lastly, my CEA does not factor in the effect of this intervention on vaccinated dogs. Since there is no effective treatment against rabies for dogs, vaccination seems to be the only way to prevent dogs from dying of rabies. Whether averting dogs’ deaths is good or bad relies on a range of factors (see sections 2.2.7 and 2.2.8). However, as is the case with the estimated number of averted human deaths, the estimated number of averted dog deaths of this intervention is very low—around 17.
Overall, while these factors could change the cost-effectiveness of the modeled intervention, I do not expect them to have a substantially large effect that would make this intervention cost-effective.
6.3.2 Assumptions under which the intervention would be cost-effective
The modeled best-case and worst-case cost-effectiveness are extremely far apart: $201 and $194,819,820 per averted DALY, respectively. This wide gap makes me doubtful about the reliability of my CEA. To strengthen the trustworthiness of my model, I analyze where this wide gap comes from and under which assumption this intervention would be cost-effective.
Comparing the estimated costs of the program in the best and worst case, it can be seen that they are not extraordinarily far apart ($923,000 vs. $1,612,000). This means that the large gap between best and worst case cost-effectiveness primarily stems from the modeled effects.
Running a basic sensitivity analysis on my model, the effects of the intervention primarily rely on the baseline dog rabies incidence, baseline dog-bite incidence and rabies transmission risk. Unfortunately, I am not very confident in the numbers cited for these factors in my model. The estimate for rabies transmission relies on a single study (see Di Quinzio & McCarthy, 2008), which is all the evidence I could find. The numbers cited for the two baseline rates come from studies based on estimates from India (see Gill et al., 2019 and John et al., 2022). The cited incidence rates are fairly low which is ultimately why the cost-effectiveness and total impact of the intervention are so low. Studies conducted in other countries report a far higher rabies incidence, for example one order of magnitude higher in Ethiopia (Jemberu et al., 2013) and up to two orders of magnitude higher in Tanzania (Cleaveland et al., 2003; note that this study is already over 20 years old). In addition, the interviewed experts (see section 5) noted that rabies cases are usually underreported and that actual rabies deaths can be up to 20 times higher than official numbers. As a result, I already increased the two baseline incidence rates in my model by 50–200%—but the intervention still turned out to be cost-ineffective.
Another indication that the two incidence rates and rabies transmission risk are a lot higher than studies shows is the following: taking the total Indian population—around 1,408,000,000 according to Our World in Data (2022)—and multiplying it by the dog rabies incidence, dog-bite incidence and rabies transmission risk would result in around 800 rabies deaths (geometric mean scenario) per year in India. In contrast, the WHO (n.d.) states that India has around 18,000–20,000 rabies deaths per year. However, even if I increase the incidence rates and rabies transmission risk to roughly result in the number of deaths similar to official numbers[8], the intervention does not turn out to be cost-effective (~12 averted deaths at ~$2,500 per averted DALY). In the best-case scenario of my CEA, the baseline rates and rabies transmission risk actually add up to a number similar to officially reported rabies deaths, but even then the intervention would not be cost-effective (~100 averted deaths at ~$200 per averted DALY). Only assuming incidence rates and a rabies transmission risk corresponding to 20 times that of the reported human rabies deaths in India would the intervention start to get cost-effective (~250 averted deaths at ~$106 per averted DALY).
This analysis shows that even assuming the most optimistic case for particularly weighty factors in my model does not make the intervention cost-effective.
7 Conclusion
Overall, my view is that oral rabies vaccination for dogs is not a recommendable intervention.
Even under the most optimistic assumptions, this intervention would only have a modest impact and not be particularly cost-effective in terms of dollars spent per averted DALY.
While there are many studies about oral rabies vaccination trials which look at campaigns’ effectiveness in terms of vaccinating a large proportion of a region’s dog population, there is no evidence on the link between rabies vaccines and reduction in human rabies cases and deaths and weak evidence on the reduction on dog rabies incidence and human dog-bite incidence.
Finally, the interviewed experts had a negative view on any work done in this space that is anything other than a large-scale nationally-coordinated implementation, ideally spanning several different countries and involving robust ongoing rabies surveillance.
Further research could look into alternative interventions in this space, such as providing sterilization or contraception for dogs, as well as lobbying for governments to invest in, build the infrastructure for and develop strategies around rabies control.
- ^
The WHO lists rabies as one of the neglected tropical diseases (NTD) since it affects already poor, marginalized and vulnerable groups (WHO, 2023).
- ^
While elimination refers to “the deliberate effort that leads to the reduction to zero of the incidence of infection caused by a specific agent in a defined geographic area”, eradication refers to “a deliberate effort that leads to the permanent reduction to zero of the worldwide incidence of infection caused by a specific agent” (Our World in Data, 2018). Rabies is not on the list of eradicable diseases and probably never will be, since so many mammals harbor it. Even in the US and Europe, where rabies is widely eliminated, there are still a few rabies cases each year (Stokstad, 2017).
- ^
The exact reason why above this vaccination coverage rabies would be effectively controlled and potentially eliminated is still not clear to me. In my expert interview (see section 4), Dr Müller and Dr Freuling mentioned that the 70% vaccination coverage already accounts for dog population turnover and other factors. So while I am fairly certain about the 70% vaccination coverage being a valid goal of an ORV campaign, I am uncertain as to why.
- ^
Dogs were considered vaccinated if they properly chewed the ORV bait (which is an indication that the vaccine sachet is perforated and the vaccine liquid released into the dog’s mouth).
- ^
When conducting subsequent ORV campaigns (e.g., in intervals of one year), it seems impossible to know which dogs were vaccinated in the previous campaign and which were not. This means baits will likely be provided to all free-roaming dogs again, leading to re-vaccinating dogs two or more years in a row. In the literature I have reviewed, it was not mentioned to what degree re-vaccinating dogs is safe. One indication that it might be safe is the study by Chanachai et al. (2021), which states that a few dogs ingested one or two (up to even six) additional baits without any adverse effects being reported.
- ^
It seems like most free-roaming dogs are owned, with only a small percentage of them being ownerless (Menghistu et al., 2018; Warembourg et al., 2021; Jibat et al., 2015).
- ^
According to the interviewed experts (see section 5), rabies deaths are often underreported and can be up to 20 times higher in Asian countries and up to 160 times higher in African countries.
- ^
I model two cases based on guesswork (both resulting in very similar cost-effectiveness and number of total deaths averted): in one case, I increase the dog rabies and dog-bite incidence by a factor of 5; in the other case, I increase both incidence rates by a factor of 2.5 and the rabies transmission risk by a factor of 4.

Great that you’ve taken the time to write this up even though the conclusion was not to recommend
Thanks for this, great work. I would like to see an analysis of this explicitly taking into account the animal welfare impacts, just using the rethink priorities values for the value of a dogs lifespan saved and the cost of the egg based treat in terms of welfare. It seems to me that the benefits of an animal vaccination campaign would largely be concentrated on the animals. As dogs are omnivores I would like to see them use plant based lures instead, as a dog owner I know that peanut butter or any number of other options would have worked well.
I agree that the animal welfare impacts of this intervention are really important to consider! I touched on them in section 2 but I unfortunately didn't have the capacity to look into the human health and animal welfare aspect of this intervention.
I also raise the issue of using egg-/meat-based baits but I honestly have no idea how their use weighs against potentially saving lots of dog lives due to vaccinating them against rabies. Interesting to hear about peanut butter potentially working well! I haven't come across this in any of the papers I've read and I'd be really curious to see how peanut butter baits would work in comparison to egg or intenstine baits in terms of acceptance and vaccine release.
Thanks for your work.
The post mentions the work done in Goa, India, I would like to highlight the cost-effectiveness of the injectable rabies vaccine reported in the paper on the intervention done. "Elimination of human rabies in Goa". (I had read this paper once and thought the numbers were impressive. feel free to criticise it)
"The estimated mean cost per death averted and cost per DALY averted from 2013 to 2019 were 14,866 USD and 526 USD, respectively. During this period, the program was estimated to result in 2,249 DALYS averted and 80 deaths averted compared to no intervention. Over a 10-year projection (2013–2023), the intervention was estimated to prevent 121 human rabies deaths and 3427 DALYS at a mean cost of 567 USD per DALY averted."
Administering injectable vaccines does pose logistical issues; They report mean vaccination coverage in the 2016 campaign as 71.8% in all sighted dogs and 60.1% in roaming dogs. In 2017, intensive methods were applied state-wide, achieving an estimated coverage of 71.7% in all dogs sighted and 53.1% in the roaming population. Would improving operational efficiency in covering the free-roaming population of dogs with the traditional rabies vaccine be considered a good recommendation?
I wonder if there might be particularly strong regional effects to this - maybe Goa had quite a large dog population, quite a lot of rabies, or quite dense dog/human populations (affecting rabies, bite, and transmission incidences).
I think there could be room for further research to identify whether there would be better-looking (sub-country) regions - though like Helene_K found, data would be difficult.
Good point! I found that my CEA model was very sensitive to dog rabies incidence. If the incidence were high enough, doing a vaccination campaign could probably become cost-effective. However, the issue is that the effect of a vaccination campaign in a specific region alone would be fleeting if surrounding areas aren't also included in the campaign (since rabid dogs and other animals likely migrate from un-vaccinated regions to vaccinated regions). I discussed this issue in more details in section 5 in my report. Ultimately, this is what kills this idea in my opinion (when considering whether to start a new charity for it): you'd have to implement this intervention on a very large scale in order to have a lasting effect.
Thanks for your comment and the paper! I've come across this paper before but didn't read it in detail. I have skimmed the paper again just now, so here are a couple of my ad-hoc thoughts:
The cost-effectiveness of the Goa campaigns seems to be a lot better than my modeled program in India. My CEA is very speculative, so it's nice to see that a retrospective CEA of this intervention yields a relatively good $-per-averted-DALY result. However, I'm not swayed by the number, since it's still far from GiveWell's cost-effectiveness bar of 100$ per averted DALYs. It would also be interesting to have a closer look at the assumptions they make in the CEA. From a brief look at the spreadsheet, they seem to equate one rabies death with ~26 YLL. This is a lot lower than my assumed ~45 DALYs per rabies death in my model (I used Founders Pledge's moral weights and roughly adapted them to the age distribution of rabies victims; also, virtually all of the DALY burden from rabies comes from YLL). This change alone would probably increase (potentially double?) the cost-effectiveness in the paper you linked but I guess it would still not pass GiveWell's bar.
Regarding the logistical issues you mention, this aligns with what I discovered in my research. As far as I know, free-roaming dogs have a higher rabies incidence than homestead dogs. But free-roaming dogs are hard to vaccinate with traditional (injectable) vaccines, since these dogs can be very shy and hard to catch. That's where oral vaccines would come in since you can simply throw a vaccination baits in front of a dog without having to come the dog. Which means that oral vaccine could be a good method to close the vaccination gap beween homestead and free-roaming dogs.
Hope this addresses some of the points you made!
Thanks for this great analysis, this matches my experience and I've often scratched my head about the potential cost-effectiveness of these kind of interventions - we even have a similar program here in Gulu Northern Uganda.
Oh, interesting to hear that there's a program in Uganda. Just googled, are you talking about The Big Fix Uganda?
Yes the big fix are very active, but there have been a bunch of other programs as well.